��Ŀ����
����������ȼ�գ������������ֹ�̬�����3.1g�ĵ����ף�P����3.2g��������ȼ�գ�����Ӧ��ľ������ų�XkJ������
��1��ͨ������ȷ����Ӧ�������ɣ��û�ѧʽ��ʾ����______������Ӧ������g��Ϊ______��
��2����֪������ȼ����ΪYkJ/mol����1mol P��O2��Ӧ���ɹ�̬P2O3�ķ�Ӧ�ȡ�H=______��
��3��д��1molP��O2��Ӧ���ɹ�̬P2O3���Ȼ�ѧ����ʽ��______��
��1��ͨ������ȷ����Ӧ�������ɣ��û�ѧʽ��ʾ����______������Ӧ������g��Ϊ______��
��2����֪������ȼ����ΪYkJ/mol����1mol P��O2��Ӧ���ɹ�̬P2O3�ķ�Ӧ�ȡ�H=______��
��3��д��1molP��O2��Ӧ���ɹ�̬P2O3���Ȼ�ѧ����ʽ��______��
��1��3.1g�ĵ����ף�P�������ʵ���Ϊ
=0.1mol��3.2g�����������ʵ���Ϊ
=0.1mol����Pԭ����Oԭ�ӵ���Ŀ֮��Ϊ0.1mol��0.1mol��2=1��2��2��5��1��2��2��3���ʷ�Ӧ����ΪP2O3��P2O5�������ʵ����ֱ�Ϊxmol��ymol����
��ã�x=0.025mol��y=0.025mol
��P2O3������Ϊ0.025mol��110g/mol=2.75g��
P2O5������Ϊ0.025mol��142g/mol=3.55g��
�ʴ�Ϊ��P2O3��P2O5��2.75g��3.55g��
��2��������ȼ����ΪYkJ/mol����1mol����ȫȼ�����ɹ�̬P2O5�ų�������ΪYkJ����Ӧ������0.025molP2O5����Ҫ��0.05mol����0.05mol��ȼ���������ɹ�̬P2O5�ų�������Ϊ0.05YkJ����Ӧ������0.025molP2O3����Ҫ��0.05mol����
����0.05mol��ȼ���������ɹ�̬P2O3�ų�������ΪXkJ-0.05YkJ=��X-0.05Y��kJ������1molP��O2��Ӧ���ɹ�̬P2O3�ų�������Ϊ��X-0.05Y��kJ��
=��20X-Y��kJ����1mol P��O2��Ӧ���ɹ�̬P2O3�ķ�Ӧ�ȡ�H=-��20X-Y��kJ/mol��
�ʴ�Ϊ��-��20X-Y��kJ/mol��
��3���ɣ�2����֪��1molP��O2��Ӧ���ɹ�̬P2O3���Ȼ�ѧ����ʽ��P��s��+
O2��g��=
P2O3��s����H=-��20X-Y��kJ/mol��
�ʴ�Ϊ��P��s��+
O2��g��=
P2O3��s����H=-��20X-Y��kJ/mol��
| 3.1g |
| 31g/mol |
| 3.2g |
| 32g/mol |
|
��ã�x=0.025mol��y=0.025mol
��P2O3������Ϊ0.025mol��110g/mol=2.75g��
P2O5������Ϊ0.025mol��142g/mol=3.55g��
�ʴ�Ϊ��P2O3��P2O5��2.75g��3.55g��
��2��������ȼ����ΪYkJ/mol����1mol����ȫȼ�����ɹ�̬P2O5�ų�������ΪYkJ����Ӧ������0.025molP2O5����Ҫ��0.05mol����0.05mol��ȼ���������ɹ�̬P2O5�ų�������Ϊ0.05YkJ����Ӧ������0.025molP2O3����Ҫ��0.05mol����
����0.05mol��ȼ���������ɹ�̬P2O3�ų�������ΪXkJ-0.05YkJ=��X-0.05Y��kJ������1molP��O2��Ӧ���ɹ�̬P2O3�ų�������Ϊ��X-0.05Y��kJ��
| 1mol |
| 0.05mol |
�ʴ�Ϊ��-��20X-Y��kJ/mol��
��3���ɣ�2����֪��1molP��O2��Ӧ���ɹ�̬P2O3���Ȼ�ѧ����ʽ��P��s��+
| 3 |
| 4 |
| 1 |
| 2 |
�ʴ�Ϊ��P��s��+
| 3 |
| 4 |
| 1 |
| 2 |

��ϰ��ϵ�д�
 ����ѧ���ʱѧ����ϵ�д�
����ѧ���ʱѧ����ϵ�д�
�����Ŀ
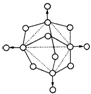 ���ڿ����г��ȼ�գ�����X�ķ��ӽṹ����ͼ��ʾ��ͼ��ʵ�߱�ʾ��ѧ����ԲȦ��ʾԭ�ӣ�������ԭ�������С��
���ڿ����г��ȼ�գ�����X�ķ��ӽṹ����ͼ��ʾ��ͼ��ʵ�߱�ʾ��ѧ����ԲȦ��ʾԭ�ӣ�������ԭ�������С��