��Ŀ����
12�������й�ʵ��������������۵�������ȷ���ǣ�������| A�� | ʯ�͵�����ʵ���У��������Ƭ�ɷ�ֹʯ��������ʱ���� | |
| B�� | �Ҵ��Ĵ�����ʵ����ͭ˿��Ҫ�������������� | |
| C�� | ��ȡ��������ʱ���Լ������˳���ǣ��ȼ����Ҵ����������������ᣬ������Ũ���� | |
| D�� | �ڵ�����Һ�м�������ϡ���ᣬˮԡ���Ⱥ��ټ����������Ƶ�������ͭ����Һ�����������ڣ�û��ש��ɫ�������ɣ�˵������û��ˮ�� | |
| E�� | ��ȥ�����л��е�������ϩ�����Խ��������ͨ��������KMnO4Һ | |
| F�� | ���ƿ��Լ���ij��ˮ�ƾ����Ƿ���ˮ | |
| G�� | ������ˮ������ֲ���ͺͿ����� |
���� A�����Ƭ���ֹ�������ã�
B���Ҵ��Ĵ�����ʵ����ͭ˿��������
C����ȡ��������ʱ����ֹ����ӷ��ͷɽ���
D���ڵ�����Һ�м�������ϡ���ᣬˮԡ���Ⱥ���ˮ�⣬����������Ӧ�ڼ�����Һ�У�
E����ϩ������KMnO4��Һ�������ɶ�����̼��
F���þƾ���ˮ����Na��Ӧ����������
G��ֲ���ͺ��в�����������������ˮ�����ӳɷ�Ӧ����
��� �⣺A�����Ƭ���ֹ�������ã�����ʯ�͵�����ʵ���У��������Ƭ�ɷ�ֹʯ��������ʱ���У���A��ȷ��
B���Ҵ��Ĵ�����ʵ����ͭ˿��������Cu�������ڷ�Ӧǰ�䣬��B����
C����ȡ��������ʱ����ֹ����ӷ��ͷɽ����Լ������˳���ǣ��ȼ����Ҵ�������������Ũ���ᣬ���������ᣬ���ȼ����Ҵ����������������ᣬ������Ũ���ᣬ��C��ȷ��
D���ڵ�����Һ�м�������ϡ���ᣬˮԡ���Ⱥ���ˮ�⣬����������Ӧ�ڼ�����Һ�У���ˮԡ���Ⱥ��ȼӼ������ԣ��ټ����������Ƶ�������ͭ����Һ�����������ڣ��۲��Ƿ���ש��ɫ�������ɣ���D����
E����ϩ������KMnO4��Һ�������ɶ�����̼�����������ʣ�Ӧ������ˮ��ϴ����ȥ�����л��е�������ϩ����E����
F���þƾ���ˮ����Na��Ӧ��������������ˮ����ͭ���Լ���ij��ˮ�ƾ����Ƿ���ˮ����F����
G��ֲ��������ˮ�����ӳɷ�Ӧʹ����ɫ��������������ˮ��Ӧ������ͬ�������𣬹�G��ȷ��
��ѡACG��
���� ���⿼�黯ѧʵ�鷽�������ۣ�Ϊ��Ƶ���㣬�漰�����ķ����ᴿ�����ʼ��顢�л�����Ʊ�ʵ���ˮ��ʵ��ȣ������������ʼ�ʵ�鼼�ܵĿ��飬ע��ʵ��������Է�������Ŀ�ѶȲ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| ������ | K+��NH4+��Fe3+��Ba2+ |
| ������ | Cl-��Br-��CO32-��HCO3-��SO32-��SO42- |
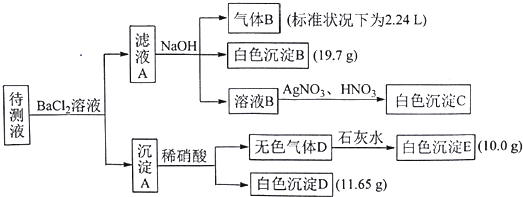
| A�� | ����ҺB�м�AgNO3��HNO3�IJ������Ƕ���ģ�����Һ������Na+�⣬һ������K+��NH4+��CO32-��HCO3-��c��K+����0.1mol•L-1 | |
| B�� | ���ɰ�ɫ����B�����ӷ���ʽΪ��Ba2++HCO3-+OH-�TBaCO3��+H2O | |
| C�� | ����ɫ����D�ǵ�һ���壬��ԭ��Һ��c��SO42-��=0.05 mol•L-1 | |
| D�� | ����ɫ����D�ǻ�����壬�����A�ijɷ���BaCO3��BaSO3��BaSO4 |
| A�� | �����ʵ�����MgCl2��Ba��OH��2��HCl��Һ��ϣ�Mg2++2OH-�TMg��OH��2�� | |
| B�� | ������ͭ�缫���CuSO4 ��Һ��2Cu2++2H2O$\frac{\underline{\;���\;}}{\;}$ 2Cu��+O2��+4H+ | |
| C�� | ����������Һ������������Һǡ�÷�Ӧ�����ԣ�H++SO42-+Ba2++OH-�TH2O+BaSO4�� | |
| D�� | ����0.1 mol���ʵ���������ϡ��Һ�м���7.8 g Na2O2��4Na2O2+4Fe2++6H2O�T4Fe��OH��3+8Na++O2�� |
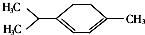 �������ǿ�ű��Чҩ������������ͼ��ʾ�л���Ҳ�������������ڸ��л�����й�˵����ȷ���ǣ�������
�������ǿ�ű��Чҩ������������ͼ��ʾ�л���Ҳ�������������ڸ��л�����й�˵����ȷ���ǣ�������| A�� | ���л����һ��ȡ�����У����������칹��8�� | |
| B�� | ���л���λ��ͬһƽ���ڵ�̼ԭ�������10�� | |
| C�� | ���л���������ˮ���ɷ����ӳɡ������;ۺϷ�Ӧ | |
| D�� | ���л�������ʽΪC5H8����ױ���Ϊͬϵ�� |
| ѡ�� | ʵ����� | ʵ������ | ʵ����� |
| A | ��ij��Һ�еμ�����NaOHϡ��Һ����ʪ��ĺ�ɫʯ����ֽ�����Թܿ� | ��ֽ������ | ԭ��Һ����NH4+ |
| B | NaHCO3��Һ��NaAlO2��Һ��� | ���ɰ�ɫ���� | ���H+��������AlO2-��CO32- |
| C | ��ˮ������Һ��ͨ������CO2 | ������ɫ���� | ���ԣ�H2CO3��H2SiO3 |
| D | ��FeCl3��Һ��ͨ������SO2���� | ��ɫ��ȥ | SO2����Ư���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
��1������FeSO4��Һʱ��Ҫ��������ϡ�����Ŀ��������FeSO4��ˮ�⣬�ж�FeSO4��Һ�Ƿ���ʵķ�����ȡ����FeSO4��Һ���Թ��У�����Һ�м���KSCN��Һ������Һ��죬��˵��FeSO4��Һ���ʣ����������ʣ�
��2��ʵ����ͨ����FeSO4��Һ�м��뱥�ͣ�NH4��2SO4��Һ��Ȼ����һϵ�в�������õ���������茶��壬���С�һϵ�в���������Ϊ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����
��3��Ϊ��ϴ����������茶���Ĵֲ�Ʒ�����з���������ʵ���d������ĸ����
a������ˮϴ b��������ˮϴ��������ˮ�Ҵ�ϴ
c����Na2SO4��Һϴ d������ˮ�Ҵ�ϴ
��4��Ϊ�˲ⶨ�������������Ʒ�Ĵ��ȣ���ȡag��Ʒ����ˮ�����Ƴ�500mL��Һ��ȡ����Һ25.00mL
��Ũ��Ϊcmol•L-1������KMnO4��Һ�ζ����ظ���������2�Σ�ʵ�������£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ���ĸ��������Һ�����/ml | 25.52 | 25.02 | 24.98 |
�ڵζ��յ�����������һ�ε��룬��Һ����ɫ��Ϊdz��ɫ����30s����ɫ����
�۸ò�Ʒ�Ĵ���Ϊ$\frac{980c}{a}$��100%���ú�a��c�Ĵ���ʽ��ʾ����
���ϱ��е�һ��ʵ���¼���������Դ��ں����Σ���ԭ�������bc������ĸ����
a��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱ���Ը��������Һ�����
b���ζ�ǰ�ζ��ܼ��������ݣ��ζ�����������
c����һ�εζ��õ���ƿ�ô�װҺ��ϴ��������������ϴ
d�������Ը�����ر�Һδ�������ữ�����������ữ��
| A�� | �����£�1LpH=13��NaOH��Һ�У���ˮ�����OH-��ĿΪ0.1NA | |
| B�� | 0.1mol${\;}_{38}^{90}$Srԭ���к�������Ϊ3.8NA | |
| C�� | 50mL12mol/L������������MnO2���ȣ�ת�Ƶ�����Ϊ0.3NA | |
| D�� | 2.0gH218O��D216O�Ļ����������������ΪNA |
 ������Ԫ��A��B��C��D��ԭ��������������������Ԫ�����ڱ��е����λ����ͼ��ʾ����֪C��A��ԭ������֮�����A��ԭ������������˵����ȷ���ǣ�������
������Ԫ��A��B��C��D��ԭ��������������������Ԫ�����ڱ��е����λ����ͼ��ʾ����֪C��A��ԭ������֮�����A��ԭ������������˵����ȷ���ǣ�������| A�� | ԭ�Ӱ뾶��D��C��B��A | |
| B�� | �����Ӱ뾶��D��A��B | |
| C�� | ���⻯����ȶ��ԣ�D��C��A | |
| D�� | ����Ԫ�ص�����������������ˮ |
| ʵ��Ŀ�� | ʵ����� | |
| A�� | �Ʊ�Fe��OH��3���� | ��NaOHŨ��Һ�μӵ�����FeCl3��Һ�� |
| B�� | ��MgCl2��Һ�Ʊ���ˮMgCl2 | ��MgCl2��Һ�������� |
| C�� | ��ȥCu���л��е�CuO | ����ϡ������Һ�����ˡ�ϴ�ӡ����� |
| D�� | �Ƚ�ˮ���Ҵ�����Ļ����� | �ֱ�������Ͷ�뵽ʢ��ˮ���Ҵ����ձ��� |
| A�� | A | B�� | B | C�� | C | D�� | D |