��Ŀ����
 ��ͼ��ʾ������ת���У�A��M��Ϊ�������ʣ�A���ճ������в���ȱ�ٵ����ʣ�MΪ�������ʣ�B��E��FΪ���嵥�ʣ�GΪ��ɫҺ�壬HΪ��ɫ���壮�����ַ�Ӧ�������������ȥ��
��ͼ��ʾ������ת���У�A��M��Ϊ�������ʣ�A���ճ������в���ȱ�ٵ����ʣ�MΪ�������ʣ�B��E��FΪ���嵥�ʣ�GΪ��ɫҺ�壬HΪ��ɫ���壮�����ַ�Ӧ�������������ȥ����1��������D�ĵ���ʽ��
��2����Ӧ�ٵĻ�ѧ����ʽΪ
��3����Ӧ�ܵ����ӷ���ʽΪ
��4����Ӧ�ݵĻ�ѧ����ʽΪ
��5����Ӧ�����ӷ���ʽΪ
��6����AlCl3��Һ�м�����������C���˷�Ӧ�Ļ�ѧ����ʽΪ
��7��I��������ˮ������Ҫԭ����
���㣺������ƶ�
ר�⣺�ƶ���
������GΪ��ɫҺ�壬ӦΪH2O��M��H2O��Ӧ���ɵ�HΪ��ɫ���壬��HΪFe3O4��MΪFe��FΪO2��EΪH2����CΪNa��DΪNa2O2��LΪNaOH��AӦΪNaCl��BΪCl2��IΪFeCl3��JΪHCl��KΪFeCl2����϶�Ӧ���ʵ����ʺ���ĿҪ��ɽ����⣮
���
�⣺GΪ��ɫҺ�壬ӦΪH2O��M��H2O��Ӧ���ɵ�HΪ��ɫ���壬��HΪFe3O4��MΪFe��FΪO2��EΪH2����CΪNa��DΪNa2O2��LΪNaOH��AӦΪNaCl��BΪCl2��IΪFeCl3��JΪHCl��KΪFeCl2��
��1��DΪNa2O2�����������Ӻ�������֮��������Ӽ�����ԭ�Ӻ���ԭ��֮����ڷǼ��Թ��ۼ�������D�ĵ���ʽΪ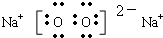 ��
��
�ʴ�Ϊ��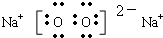 ��
��
��2����Ӧ��Ϊ������ڵ�NaCl�ķ�Ӧ������ʽΪ2NaCl
2Na+Cl2����
�ʴ�Ϊ��2NaCl
2Na+Cl2����
��3����Ӧ�ܹ���������ˮ�ķ�Ӧ����Ӧ�����ӷ���ʽΪ2Na2O2+2H2O�T4Na++4OH-+O2����
�ʴ�Ϊ��2Na2O2+2H2O�T4Na++4OH-+O2����
��4����Ӧ��Ϊ����ˮ�ķ�Ӧ����Ӧ�ķ���ʽΪ3Fe+4H2O��g��
Fe3O4+4H2��
�ʴ�Ϊ��3Fe+4H2O��g��
Fe3O4+4H2��
��5����Ӧ��ΪFe3O4������ķ�Ӧ����Ӧ�����ӷ���ʽΪFe3O4+8H+�T2Fe3++Fe2++4H2O��
�ʴ�Ϊ��Fe3O4+8H+�T2Fe3++Fe2++4H2O��
��6����AlCl3��Һ�м�����������Na������������������������Ӧ�����ӷ���ʽΪ2AlCl3+6Na+6H2O�T2Al��OH��3��+3H2��+6NaCl��
�ʴ�Ϊ��2AlCl3+6Na+6H2O�T2Al��OH��3��+3H2��+6NaCl��
��7��IΪFeCl3������ˮ��ˮ�����ɾ��������Ե������������壬�����ھ�ˮ���ʴ�Ϊ����ˮ��ˮ�����ɾ��������Ե������������壮
��1��DΪNa2O2�����������Ӻ�������֮��������Ӽ�����ԭ�Ӻ���ԭ��֮����ڷǼ��Թ��ۼ�������D�ĵ���ʽΪ
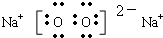 ��
���ʴ�Ϊ��
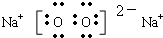 ��
����2����Ӧ��Ϊ������ڵ�NaCl�ķ�Ӧ������ʽΪ2NaCl
| ||
�ʴ�Ϊ��2NaCl
| ||
��3����Ӧ�ܹ���������ˮ�ķ�Ӧ����Ӧ�����ӷ���ʽΪ2Na2O2+2H2O�T4Na++4OH-+O2����
�ʴ�Ϊ��2Na2O2+2H2O�T4Na++4OH-+O2����
��4����Ӧ��Ϊ����ˮ�ķ�Ӧ����Ӧ�ķ���ʽΪ3Fe+4H2O��g��
| ||
�ʴ�Ϊ��3Fe+4H2O��g��
| ||
��5����Ӧ��ΪFe3O4������ķ�Ӧ����Ӧ�����ӷ���ʽΪFe3O4+8H+�T2Fe3++Fe2++4H2O��
�ʴ�Ϊ��Fe3O4+8H+�T2Fe3++Fe2++4H2O��
��6����AlCl3��Һ�м�����������Na������������������������Ӧ�����ӷ���ʽΪ2AlCl3+6Na+6H2O�T2Al��OH��3��+3H2��+6NaCl��
�ʴ�Ϊ��2AlCl3+6Na+6H2O�T2Al��OH��3��+3H2��+6NaCl��
��7��IΪFeCl3������ˮ��ˮ�����ɾ��������Ե������������壬�����ھ�ˮ���ʴ�Ϊ����ˮ��ˮ�����ɾ��������Ե������������壮
���������⿼��������ƶϣ�Ϊ�߿��������ͣ�������ѧ���ķ����������ۺ����û�ѧ֪ʶ��������ע�����������ʵ����ʣ�����ȷ��д��ط�Ӧ�����ӷ���ʽ��ѧ����ʽ���ѶȲ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
������������������ȷ���ǣ�������
| A�������в�һ��������Ԫ�� |
| B��������һ��������Ԫ�� |
| C���ڷ�Ӧ����ʧ���ӵ����� |
| D���ڷ�Ӧ�л��ϼ����ߵ����� |
���л�ѧ������ȷ���ǣ�������
| A��CH2CH2--��ϩ�Ľṹ��ʽ |
| B��C2H6--����Ľṹʽ |
C�� -���ĵ���ʽ -���ĵ���ʽ |
D�� --���ķ���ʽ --���ķ���ʽ |
�����£�0.1mol?L-1ijһԪ�ᣨHA����Һ��
=1��10-8������������ȷ���ǣ�������
| c(OH-) |
| c(H+) |
| A����Һ��ˮ�������c��H+��=10-10mol?L-1 |
| B��������Һ�м���һ����CH3COONa������ˮϡ�ͣ���Һ��c��OH-�������� |
| C����0.05 mol?L-1NaOH��Һ�������Ϻ�������Һ������Ũ�ȴ�С��ϵΪc��A-����c��Na+����c��OH-����c��H+�� |
| D����Һ��c��H+��+c��A-��=0.1 mol?L-1 |
�������ӷ���ʽ��ȷ���ǣ�������
| A��ʯ��ʯ���ڴ�����Һ�У�CaCO3+2CH3COOH�T2CH3COO-+Ca2++CO2��+H2O |
| B����������ͭ��Һ��Ӧ��Cu2++2Na�T2Na++Cu |
| C��������м����ϡ���3Fe+8H++2NO3-�T3Fe2++2NO��+4H2O |
| D����������Һ�м������������������Һ��Al3++2SO42-+2Ba2++3OH-�T2BaSO4��+Al��OH��3�� |
 ����ͼ����ѧ��ѧ�г�������֮���һЩ��Ӧ��ϵ�����в��ֲ���δд����������X�ǰ�ɫ���壬H�Ǻ�ɫ���嵥�ʣ�B��Һ�壬A��C��D��E��F��Ϊ���壬��C��������ʯ�������1mol X���ȷֽ�õ�A��B��C��1mol��
����ͼ����ѧ��ѧ�г�������֮���һЩ��Ӧ��ϵ�����в��ֲ���δд����������X�ǰ�ɫ���壬H�Ǻ�ɫ���嵥�ʣ�B��Һ�壬A��C��D��E��F��Ϊ���壬��C��������ʯ�������1mol X���ȷֽ�õ�A��B��C��1mol��