��Ŀ����
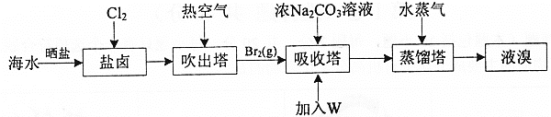
����Ŀ����ˮɹ�κ����±�к�Br-������ȡBr2��������ͼ:
��֪:��3Br2+3CO![]() = 5Br- +BrO
= 5Br- +BrO![]() +3CO2����5Br- +BrO
+3CO2����5Br- +BrO![]() +6H+ =3Br2 +3H2O
+6H+ =3Br2 +3H2O
����˵������ȷ����
A.��ˮɹ����ʵ����Ԫ�صĸ���
B.ͨ��Cl2������Ӧ: 2Br- +Cl2= Br2 +2Cl-
C.�������м���W��Һ��õ�Br2��W����������
D.��ˮ��Br-��Ũ��ԼΪ66mg��L-1 �����ù�������ȡ��Ϊ60%��1m3��ˮ���Ƶ�39.6g Br2
���𰸡�C
��������
A����ˮɹ�κ�õ���±ˮ��Br-��Ũ�ȴ��������ʵ����Ԫ�صĸ�����A��ȷ��
B��ͨ��Cl2���Ŀ�ľ��ǽ�Br-����ΪBr2�ʷ�����Ӧ�����ӷ���ʽΪ��2Br-+Cl2= Br2+2Cl-��B��ȷ��
C���������ǿ�����ԣ�����Br-��Ӧ�����ж��к�������NO���������м���W��Һ��õ�Br2��W�����������ᣬ��Ӧ����ϡ���ᣬ������Ӧ�ڵõ�Br2��C����
D�����������غ��֪����ˮ��Br-��Ũ��ԼΪ66mg��L-1�����ù�������ȡ��Ϊ60%��1m3��ˮ���Ƶ�66mg��L-1��1000L��60%��10-3g/mg=39.6g Br2��D��ȷ��
�ʴ�Ϊ��C��

 �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д�����Ŀ������ʵ�鷽�����ܴﵽʵ��Ŀ����
ʵ��Ŀ�� | ʵ�鷽�� | |
A | ֤�������鷢����ȥ��Ӧ����ϩ���� | ���Թ��м����������������NaOH���Ҵ���Һ�����ȣ���������������ͨ��������Ȼ�̼��Һ |
B | ����±������±ԭ�ӵ����� | ��������������������Һ���ȣ�ȡ��ȴ��ӦҺ�������еμ������ữ�����������Һ |
C | ���� | �� |
D | ��֤����Һ���� | ����Ӧ�����Ļ��������ͨ�����Ȼ�̼��Һ��ͨ�� |
A.AB.BC.CD.D
����Ŀ���������ʹ�������ǿ����������ܵ���Ҫ�о�����
(1)![]() ��һ�ִ�����ϣ�����
��һ�ִ�����ϣ�����![]() ��
��![]() ��Ӧ�Ƶá�
��Ӧ�Ƶá�
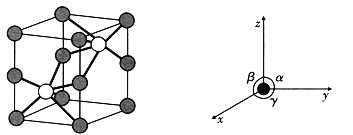
����̬Clԭ���У�����ռ�ݵ���ߵ��Ӳ����Ϊ ______���õ��Ӳ���е�ԭ�ӹ����Ϊ _______��
��Li��B��HԪ�صĵ縺���ɴ�С������˳��Ϊ ___________��
(2)�����⻯���Ǿ������÷�չǰ���Ĵ�����ϡ�
��LiH�У����Ӱ뾶��Li+ ___________(����>����=������<��)H-��
��ij��������Ƕ����ڽ���Ԫ��M���⻯�M�IJ��ֵ����������ʾ��
|
|
|
|
|
738 | 1451 | 7733 | 10540 | 13630 |
��M�� ______________ (��Ԫ������)��