��Ŀ����
2�������к��зḻ�ĵ⣮Ϊ�˴Ӻ�������ȡ�⣬ij�о���ѧϰС����Ʋ�����������ʵ�飺
����д���пհף�
��1�������ȼ�ոɺ���ʱ������Ҫ���żܡ������Ǻ;ƾ����⣬����Ҫ�õ���ʵ��������B
A���ձ� B������ C�������� D�Թ�
��2������۵�ʵ����������ǹ��ˣ����������ȡ��ˮ�еĵ⣬�ò����ʵ�������������ȡ��Һ��
��3������ܷ�Ӧ�����ӷ���ʽ��2I-+MnO2+4H+=Mn2++I2+2H2O��
���� ���������յõ������ң�������Һ����Ϊ�����ͺ���������ҺӦѡ����˵ķ���������������Һͨ������Cl2��Ϊ�˽������������ɵ��ʵ⣬���ӷ���ʽΪ2I?+Cl2=I2+2Cl-�����õ��������л��ܼ���������������ȡ�����������ķ����������л���Դ˽����⣮
��� �⣺���������յõ������ң�������Һ����Ϊ�����ͺ���������ҺӦѡ����˵ķ���������������Һͨ������Cl2��Ϊ�˽������������ɵ��ʵ⣬���ӷ���ʽΪ2I?+Cl2=I2+2Cl-�����õ��������л��ܼ���������������ȡ�����������ķ����������л��
��1�����պ�����Ҫ����װ�ã����Բ�������պ���ʱ����Ҫ�õ����żܣ����������������Լ��ƾ��ƣ��ʴ�Ϊ��B��
��2��������Ƿ�������Һ�壬��ʵ�����Ϊ���ˣ������Ŀ���ǴӺ��ⱽ��Һ�з�������ʵ�ͻ��ձ���
���������ȡ��ˮ�еĵ⣬��������ڱ����л��ܼ���������ȡ��Һ�ķ������룬
�ʴ�Ϊ�����ˣ���ȡ��Һ��
��3�������������������¿ɱ�MnO2�������ɵ��ʵ⣺���ӷ���ʽΪ��2I-+MnO2+4H+=Mn2++I2+2H2O���ʴ�Ϊ��2I-+MnO2+4H+=Mn2++I2+2H2O��
���� ���⿼�����ʵķ��롢�ᴿ�Լ��Ʊ�ʵ�鷽������ƣ�Ϊ��Ƶ���㣬������ѧ���ķ�����ʵ�������Ŀ��飬��Ŀ�Ѷ��еȣ�ע�����ʵ�������Ҫ���ע�����

 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д����������ķ���װ�� ���ƶ�����̼�ķ���װ�� ����ˮ���������������������� ������������������
| A�� | �ڡ��ۻ�� | B�� | �ڡ��ۻ�� | C�� | �١��ۻ�� | D�� | �١��ۻ�� |
| A�� | ��������ȷֽ������� | B�� | ���ȷ�Ӧ | ||
| C�� | �ƺ�ˮ��Ӧ | D�� | ʵ�����Ʊ����� |
| A�� |  ��1LŨ�Ⱦ�Ϊ0.1mol/L��Ba��OH��2��NaAlO2�����Һ����μ���0.1mol/l��H2SO4��Һ | |
| B�� | 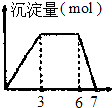 ��0.1mol/L��AlCl3��0.3mol/LNH4Cl��1L�����Һ����μ���0.1mol/l��NaOH��Һ | |
| C�� | 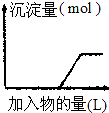 ���ռ���Һ����μ���������Һ | |
| D�� | 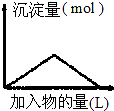 ��Ba��OH��2��Һ����ͨ�������̼���� |
| A�� | N2��H2��������ȡ���� | B�� | ����NH4Cl��ȡ���� | ||
| C�� | ��Ũ��ˮ�������ƹ����ϵμ� | D�� | ��NH4Cl��Һ��NaOH��Һ��� |
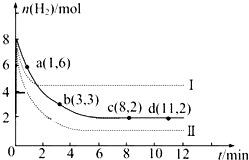 ��ҵ�ϡ��̶���������CO2����Ч�ؼ��ᡰ���ҡ�ЧӦ������CO2����ȼ�ϼ״���
��ҵ�ϡ��̶���������CO2����Ч�ؼ��ᡰ���ҡ�ЧӦ������CO2����ȼ�ϼ״���