��Ŀ����
8�����м������ʵ��۵㣨�棩���ݣ�| A�� | B�� | C�� | D�� |
| ���ʯ��3550 | Li��181 | HF��-83 | NaCl��801 |
| �辧�壺1410 | Na��98 | HCl��-115 | KCl��776 |
| ���壺2300 | K��64 | HBr��-89 | RbCl��718 |
| �������裺1723 | Rb��39 | HI��-51 | CaCl��645 |
��2��B�龧�干ͬ�����������Ǣ٢ڢۢܣ�����ţ���
���н������� �ڵ����ԡ� �۵����ԡ� ����չ��
��3��C����HF�۵㷴��������HF���Ӽ����γ���������ۻ�ʱ��Ҫ���ĵ��������࣮
��4��D�龧����ܾ��е������Ǣڢܣ�����ţ���
��Ӳ��С�� ��ˮ��Һ�ܵ��� �۹����ܵ��硡������״̬�ܵ���
��5��D�龧����۵�NaCl��KCl����ԭ�����Ϊ��NaCl��KCl��Ϊ���Ӿ��壬r��Na+����r��K+�������������������ͬ������£��뾶ԽС��������Խ���۵��Խ�ߣ�
���� ��1���������ʵ���ɺ��۵��֪A������ԭ�Ӿ��壬B�����ڽ������壬C�����ڷ��Ӿ��壬D���������Ӿ��壻
��2��B������Ϊ�������壬���н�����ͨ�ԣ�
��3������HF�д������������HF�ķе�������⻯��ķе�ߣ�
��4��D������Ϊ���Ӿ��壬�������Ӿ���������жϣ�
��5�����Ӿ���ľ����ܴ�Сȡ�������Ӱ뾶�ĵ�ɵ����أ����Ӱ뾶ԽС�����Խ�࣬������Խ�����Ӿ�����۵�Խ�ߣ�
��� �⣺��1��A���۵���ߣ�����ԭ�Ӿ��壬ԭ�Ӿ���Ĺ�����Ϊԭ�ӣ�����������Ϊ���ۼ���
�ʴ�Ϊ��ԭ�ӣ����ۼ���
��2��B������Ϊ���������н����������ԡ������ԡ���չ�ԣ�
�ʴ�Ϊ���٢ڢۢܣ�
��3������HF�д������������HF�ķе�������⻯��ķе�ߣ�
�ʴ�Ϊ��HF���Ӽ����γ���������ۻ�ʱ��Ҫ���ĵ��������ࣻ
��4��D������Ϊ���Ӿ��壬��Ӳ�ȴ�ˮ��Һ�ܵ��硢���岻�ܵ��������״̬�ܵ�������ʣ�
�ʴ�Ϊ���ڢܣ�
��5��NaCl��KCl��Ϊ���Ӿ��壬r��Na+����r��K+�������������������ͬ������£��뾶ԽС��������Խ���۵��Խ�ߣ������۵㣺NaCl��KCl��
�ʴ�Ϊ��NaCl��KCl��Ϊ���Ӿ��壬r��Na+����r��K+�������������������ͬ������£��뾶ԽС��������Խ���۵��Խ�ߣ�
���� ͨ����ȡ�������������жϳ���������ͼ���������ʣ�Ӧ���������HF���۵㷴�������þ����ܵĴ�С�������Ӿ����۵�ߵ͵�ԭ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | C2H4 ��C2H6 | B�� | CH4��C3H6 | C�� | C3H8��C2H4 | D�� | CH4 ��C2H4 |
| A�� | 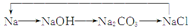 | B�� |  | ||
| C�� |  | D�� |  |
| A�� | ���� | B�� | 𤽺��ά | C�� | ������ά | D�� | ������ά |
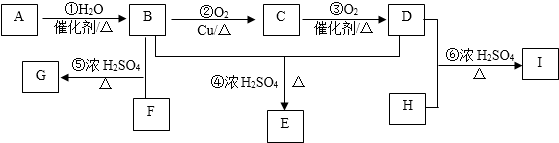
 ��
��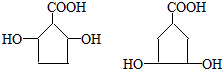 ��
��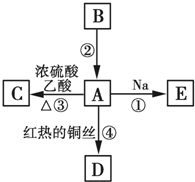 A��B��C��D��E��Ϊ�л������A�ǻ�ѧʵ����������л����������ˮ����������ζ��B�IJ����ɺ���һ������ʯ�ͻ�����չ��ˮƽ���й����ʵ�ת����ϵ��ͼ��ʾ��
A��B��C��D��E��Ϊ�л������A�ǻ�ѧʵ����������л����������ˮ����������ζ��B�IJ����ɺ���һ������ʯ�ͻ�����չ��ˮƽ���й����ʵ�ת����ϵ��ͼ��ʾ�� CH3COOC2H5+H2O
CH3COOC2H5+H2O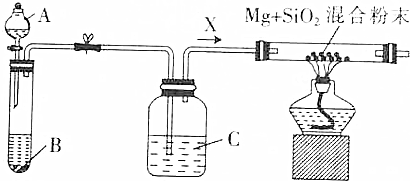
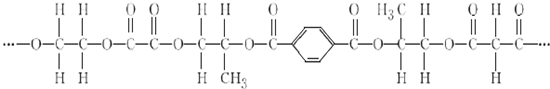
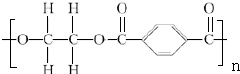 +2nH2O��
+2nH2O��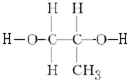 ��
��