��Ŀ����
�����£���0.1mol��L-1��������μ��뵽20mL0.1mol?L-1��ˮ�У������Һ��pH��������������仯��ͼ��ʾ������˵����ȷ���ǣ�������
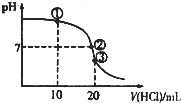
| A������Һ��c��Cl-����c(NH4+)��c��OH-����c��H+�� |
| B������Һ�У�c��NH 4+��=c��Cl-��=c��OH-��=c��H+�� |
| C������Һ�У�c��Cl-��+c��H+��=c(NH4+)+c��OH-�� |
| D���ζ������п��ܳ��֣�c��NH3H?2O����c(NH4+)��c(OH-)��c��Cl-����c��H+�� |

A������Һ�е�����Ϊ�����ʵ���Ũ�ȵİ�ˮ���Ȼ�泥���ˮ�ĵ���̶ȴ���笠����ӵ�ˮ��̶ȣ�������Һ�ʼ��ԣ�c��OH-����c��H+�������ݵ���غ��c��Cl-����c��NH4+������A����
B������Һ�����ԣ�c��OH-��=c��H+�������ݵ���غ��c��Cl-��=c��NH4+������Ϊˮ�ĵ������������c��Cl-����c��OH-������B����
C�������������Ȼ�泥���Һ�����ԣ�c��OH-����c��H+������Һ�ʵ����ԣ�����c��NH4+��+c��H+��=c��Cl-��+c��OH-��������c��Cl-����c��NH4+������c��Cl-��+c��H+����c��NH4+��+c��OH-������C����
D������Һ�а�ˮ����ԶԶ��������ʱ�����ܳ���c��NH3H?2O����c��NH4+����c��OH-����c��Cl-����c��H+������D��ȷ��
��ѡD��
B������Һ�����ԣ�c��OH-��=c��H+�������ݵ���غ��c��Cl-��=c��NH4+������Ϊˮ�ĵ������������c��Cl-����c��OH-������B����
C�������������Ȼ�泥���Һ�����ԣ�c��OH-����c��H+������Һ�ʵ����ԣ�����c��NH4+��+c��H+��=c��Cl-��+c��OH-��������c��Cl-����c��NH4+������c��Cl-��+c��H+����c��NH4+��+c��OH-������C����
D������Һ�а�ˮ����ԶԶ��������ʱ�����ܳ���c��NH3H?2O����c��NH4+����c��OH-����c��Cl-����c��H+������D��ȷ��
��ѡD��

��ϰ��ϵ�д�
 �ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
�ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
�����Ŀ