��Ŀ����
��10�֣�����500mL 0.5mol/L��NaOH��Һ���Իش��������⡣
(1)��ҪNaOH���������Ϊ ��
(2)ijͬѧ��������ƽ����һ��С�ձ�������������ǰ��������ڱ�ߵ���̶ȴ�����ƽ��ֹʱ����ָ���ڷֶ��̵�ƫ��λ�ã���ʱ��ߵ����̽� ������ڡ����ڡ����ұߵ����̣���ʹ��ƽƽ�⣬�����еIJ���Ӧ ���ٶ����ճƵ�С�ձ�������Ϊ ���32.6g����32.61g������
(3)���Ʒ������������������������裬��ȷ��˳���� ��
����ʢ��NaOH���ձ��м���200mL����ˮʹ���ܽ⣬����ȴ�����¡�
�ڼ���������ƿ�м�����ˮ��Һ���̶���1c m��2cm��
m��2cm��
�۽�NaOH��Һ�ز�����ע��500mL����ƿ�С�
�����ձ��м�������������ˮ��С��ϴ��2��3�κ��� ������
������ ƿ��
ƿ��
�ݸ��ý�ͷ�ιܼ�����ˮ���̶��ߣ��Ӹ�ҡ�ȡ�
(4)ijѧ��ʵ������NaOH��Һ��Ũ��Ϊ0.48mol/L��ԭ������ǣ� ��
(1)��ҪNaOH���������Ϊ ��
(2)ijͬѧ��������ƽ����һ��С�ձ�������������ǰ��������ڱ�ߵ���̶ȴ�����ƽ��ֹʱ����ָ���ڷֶ��̵�ƫ��λ�ã���ʱ��ߵ����̽� ������ڡ����ڡ����ұߵ����̣���ʹ��ƽƽ�⣬�����еIJ���Ӧ ���ٶ����ճƵ�С�ձ�������Ϊ ���32.6g����32.61g������
(3)���Ʒ������������������������裬��ȷ��˳���� ��
����ʢ��NaOH���ձ��м���200mL����ˮʹ���ܽ⣬����ȴ�����¡�
�ڼ���������ƿ�м�����ˮ��Һ���̶���1c
 m��2cm��
m��2cm���۽�NaOH��Һ�ز�����ע��500mL����ƿ�С�
�����ձ��м�������������ˮ��С��ϴ��2��3�κ���
 ������
������ ƿ��
ƿ���ݸ��ý�ͷ�ιܼ�����ˮ���̶��ߣ��Ӹ�ҡ�ȡ�
(4)ijѧ��ʵ������NaOH��Һ��Ũ��Ϊ0.48mol/L��ԭ������ǣ� ��
| A��ʹ����ֽ�����������ƹ��� | B������ƿ��ԭ��������������ˮ |
| C���ܽ��õ��ձ�δ�����ϴ�� | D���ý�ͷ�ιܼ�ˮ����ʱ���ӿ̶��� |
��10�֣�
(1) 10.0g ��2�֣�
(2) ���� ����ߵ�ƽ����ĸ���������ұ���ĸ������ֱ����ƽƽ�� 32.6g ��3�֣�
(3) �٢ۢܢڢݣ�˳��ȫ��2�֣�
(4) A C D ��3�֣�
(1) 10.0g ��2�֣�
(2) ���� ����ߵ�ƽ����ĸ���������ұ���ĸ������ֱ����ƽƽ�� 32.6g ��3�֣�
(3) �٢ۢܢڢݣ�˳��ȫ��2�֣�
(4) A C D ��3�֣�
��

��ϰ��ϵ�д�
 ������ÿ�ʱ��ҵϵ�д�
������ÿ�ʱ��ҵϵ�д�
�����Ŀ
 ______������ţ�����ȱ�ٵ������� ��
______������ţ�����ȱ�ٵ������� ��
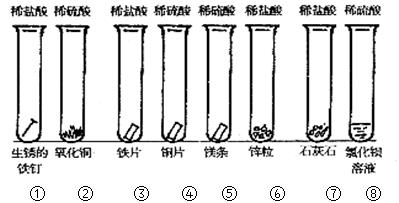
 ��
��
 ���ʵ���Ũ�ȵ�NaOH��Һʱ�����в�����������ҺŨ�Ȼ����ʲôӰ�죿���ƫ�ߡ�����ƫ�͡�����Ӱ�족��
���ʵ���Ũ�ȵ�NaOH��Һʱ�����в�����������ҺŨ�Ȼ����ʲôӰ�죿���ƫ�ߡ�����ƫ�͡�����Ӱ�족�� �IJ��������ҳ�����ͼʾ����ȷ��ʵ����� �� ��
�IJ��������ҳ�����ͼʾ����ȷ��ʵ����� �� ��