��Ŀ����
4���������һ�ֹ㷺Ӧ����ҽҩ��ʳƷ����ˮ�����ȶ����ҵ����Ҫ������Ʒ���������к���SiO2��Al2O3������Fe2O3����ͼ���Ը�����Ϊԭ���Ʊ������������������Ĺ������̣�
��ش��������⣺
��1�����˲����л�ʹ�õ��IJ����������ձ�����������©����
��2��������������Ҫ�ʵ�������Һ��pH��������2����Ҫ�ɷ�ΪFe��OH��3���ѧʽ������Ҫ������������Һ���Ƿ���Fe3+���ɲ�ȡ��ʵ�����������Ϊȡ�������������Һ���Թ��У��μӼ���KSCN��Һ������Һ��ΪѪ��ɫ��Fe3+������Fe3+��
��3��ʵ��������������ֻ����NH4+��Al3+��SO42-���������ӣ�ͬʱ����һ����Ŀ�Ľᾧˮ��ijʵ��С��Ϊ��һ���ⶨ������Ļ�ѧʽ������ȡ9.06g����������Һ��Ȼ����μ���0.2mol/LNaOH��Һ�������������ij��������ͼ����NaOH��Һ�����ϵ����ͼ��ʾ��

2��ͼ��BC �ζ�Ӧ�Ļ�ѧ����ʽAl��OH��3+NaOH=NaAlO2+2H2O��
�ڸ�����ͼ���ṩ����Ϣ��ͨ���������������Ļ�ѧʽ����д��������̣�
���� ��������Ũ�����ܽ⣬Al2O3��Fe2O3��Ӧ�õ�Al2��SO4��3��Fe2��SO4��3��SiO2�������ᷴӦ�����˵õ�����1ΪSiO2����Һ�к���Al2��SO4��3��Fe2��SO4��3��ʣ������ᣬ�����Ŀ�������
��1�����˲����л�ʹ�õ��IJ����������ձ�����������©����
��2���������ܺ�KSCN��Һ��Ӧ����Ѫ��ɫ��Һ��
��3���ٳ������ٵķ�Ӧ�����������ܽ�������������Һ�ķ�Ӧ��
�ڸ���ͼ��B��C��֪�ܽ���������������n��Al3+����A��B��NH4++OH-��NH3��H2O��n��NH4+�������ݸ��ݵ���غ� �����n��SO42-�������������غ� �����n��H2O�����ɵ�������Ļ�ѧʽ��
��� �⣺��1�����˲����л�ʹ�õ��IJ����������ձ�����������©����
�ʴ�Ϊ��©����
��2������2����Ҫ�ɷ�ΪFe��OH��3���������ܺ�KSCN��Һ��Ӧ����Ѫ��ɫ��Һ������Ҫ������������Һ���Ƿ���Fe3+��Ӧ��ȡ��ʵ�鷽��Ϊȡ�������������Һ���Թ��У��μӼ���KSCN��Һ������Һ��ΪѪ��ɫ��Fe3+������Fe3+����
�ʴ�Ϊ��Fe��OH��3��ȡ�������������Һ���Թ��У��μӼ���KSCN��Һ������Һ��ΪѪ��ɫ��Fe3+������Fe3+��
��3���ٳ������ٹ��̣���BC�Σ��з�����Ӧ���������������ܽ�������������Һ�еķ�Ӧ����Ӧ�Ļ�ѧ����ʽΪAl��OH��3+NaOH=NaAlO2+2H2O��
�ʴ�Ϊ��Al��OH��3+NaOH=NaAlO2+2H2O��
�ڸ���ͼ��B��C��֪�ܽ���������������ȥ100mL����������Һ����Al��OH��3+NaOH=NaAlO2+2H2O �����n��Al3+��=0.2mol/L��0.1L=0.02mol��A��B���������䣬˵��NH4++OH-��NH3��H2O���ɴ� �����n��NH4+��=0.2mol/L��0.1L=0.02mol�����ݵ���غ� �����n��SO42-��=$\frac{0.02mol+0.02mol��3}{2}$=0.04mol�����������غ� �����n��H2O��=$\frac{9.06g-0.02mol��27g/mol-0.02mol��18g/mol-0.04mol��96g/mol}{18g/mol}$=0.24mol����n��NH4+����n��Al3+����n��SO42-����n��H2O��=0.02��0.02��0.04��0.24=1��1��2��12������������Ļ�ѧʽΪNH4Al��SO4��2•12H2O��
�ʴ�Ϊ��NH4Al��SO4��2•12H2O��
���� ���⿼�����ʷ�����ᴿ��Ϊ��Ƶ���㣬���ؿ���ѧ������������ʵ��������������ȣ���ȷ����ͼ�з����ķ�Ӧ�������������������������ǽⱾ��ؼ���֪������������;�������غ���м��㣬��Ŀ�Ѷ��еȣ�

 25��ʱ��������ĵ���ƽ�ⳣ�������
25��ʱ��������ĵ���ƽ�ⳣ�������| ��ѧʽ | CH3COOH | H2CO3 | HClO |
| ����ƽ�ⳣ�� | 1.8��10-5 | K1 4.3��10-7 K2 5.6��10-11 | 3.0��10-8 |
��2����ͬ�����£������Ϊ200mL��Ũ�Ⱦ�Ϊ0.1mol•L-1��CH3COOH��aq����HCl��aq���ֱ���100mL 0.2mol•L-1NaOH��Һ��Ӧ���ų��������ٵ��Ǵ��� �������ƣ���
��3�������������ӽ�����������ɴ�С��˳����a��b��d��c������ţ���
a��CO32-b��ClO- c��CH3COO-d��HCO3-
��4�������������Һ��ͨ������������̼��д���ù����з�Ӧ�����ӷ���ʽ��ClO-+CO2+H2O=HCO3-+HClO��
��5����һ���¶��£��� a������ b������ c�����������ᣬ������c��H+����ͬ�������ͬʱ��ͬʱ������״���ܶȡ�������ȫ��ͬ��п������������ͬ�����H2����ͬ״��������ʼʱ��Ӧ���ʵĴ�С��ϵΪa=b=c������ţ�����Ӧ����ʱ��ij��̹�ϵ��a=b��c������ţ���
��6����ͬ�����£������Ϊ10mL��c��H+��Ϊ0.01mol•L-1�Ĵ�����Һ��HX��Һ�ֱ��ˮϡ����1000mL��ϡ������c��H+���仯��ͼ��ʾ��
��HX�ĵ���ƽ�ⳣ���ڣ�����ڡ��������ڡ���С�ڡ�������ĵ���ƽ�ⳣ����
A����ȡ20mL����������Һע��ྻ����ƿ��������2��3�η�̪��
B���ñ���Һ��ϴ�ζ���2��3�Σ�
C����ʢ�б���Һ�ļ�ʽ�ζ��̶ܹ��ã����ڵζ��ܼ���ʹ֮������Һ��
D��ȡ��NaOH��Һע���ʽ�ζ������̶�0����2��3cm��
E������Һ����0��0���¿̶ȣ����¶�����
F������ƿ���ڵζ��ܵ����棬�ñ�NaOH��Һ�ζ����յ㲢���µζ���Һ��Ŀ̶ȣ�
�ʹ�ʵ�������գ�
��1����ȷ���������˳���ǣ��������ĸ��д��ABDCEF��
��2������B���������Ŀ���dz�ȥ���ڵζ��ܱ��ϵ�ˮ����ֹˮϡ�ͱ���Һ��
��3��ʵ���������ֿ��ƻ������۾�ע����ƿ����Һ����ɫ�仯��ֱ���ζ��յ㣮�жϵ����յ����������Һ����ɫ����ɫ��dz���ұ��ְ�����ڲ���ɫ��
��4��ijѧ������3��ʵ��ֱ��¼�й����������
| �ζ� ���� | ������Һ�������mL�� | 0.100 0mol•L-1NaOH�������mL�� | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ�����mL�� | ||
| ��һ�� | 20.00 | 0.00 | 26.11 | 26.11 |
| �ڶ��� | 20.00 | 1.56 | 30.30 | 28.74 |
| ������ | 20.00 | 0.22 | 26.31 | 26.09 |
��5����0.1000mol•L-1 NaOH��Һ�ζ�0.1000mol•L-1���ᣬ��ﵽ�ζ����յ�ʱ���������1��NaOH��Һ��1����Һ�����ԼΪ0.05mL����������ˮ��50mL��������Һ��pH����10
��6��������Щ������ʹ�ⶨ���ƫ��B��C��E������ţ���
A����ƿ����Һ����ɫ�ո�����ɫ��Ϊdz��ɫ��ֹͣ�ζ�
B����ʽ�ζ���������ˮϴ��������ע���Һ
C���ζ�ǰ��ʽ�ζ��ܼ������δ�ų����ζ���������ʧ
D���ζ�ǰ������ȷ���ζ����ӵζ��ܶ���
E��ʵ���У��ô�ʢװ����Һ��ϴ��ƿ��


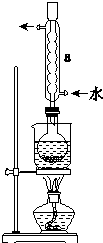 Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ���
Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ���
 2SO2��g��+O2��g��?2SO3��g����H=a kJ•mol-1����Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2��g����ȫת��Ϊ1mol SO3��g������99kJ����ش�
2SO2��g��+O2��g��?2SO3��g����H=a kJ•mol-1����Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2��g����ȫת��Ϊ1mol SO3��g������99kJ����ش�
