��Ŀ����
���Ϲ����к���ƻ���ᣨMLA���������ʽΪC4H6O5��0.1molƻ����������NaHCO3��Һ��Ӧ�ܲ���4.48L CO2����״������ƻ������ˮ������ʹ��ˮ��ɫ�IJ��ƻ���ᾭ�ۺ����ɾ�ƻ���ᣨPMLA����

��1��д���������ʵĽṹ��ʽ��B ��D ��
��2��A�к��еĹ����ŵ����ƣ� ��MLA�ĺ˴Ź��������� ��壮
��3����MLA������ͬ�����ŵ�ͬ���칹���� �֣�
��4��д��E��Fת���Ļ�ѧ����ʽ ���÷�Ӧ�ķ�Ӧ�����ǣ� ��
��5������ת����ϵ�в���ۺܵ͢�˳���ܷ�ߵ��� ����ܡ����ܡ���˵�����ɣ� ��
��6��PMLA�������õ����������ԣ�������Ϊ��������ߵȲ���Ӧ��������ҽҩ�������������������������ˮ��Ļ�ѧ����ʽΪ ��

��1��д���������ʵĽṹ��ʽ��B
��2��A�к��еĹ����ŵ����ƣ�
��3����MLA������ͬ�����ŵ�ͬ���칹����
��4��д��E��Fת���Ļ�ѧ����ʽ
��5������ת����ϵ�в���ۺܵ͢�˳���ܷ�ߵ���
��6��PMLA�������õ����������ԣ�������Ϊ��������ߵȲ���Ӧ��������ҽҩ�������������������������ˮ��Ļ�ѧ����ʽΪ
���㣺�л���ĺϳ�
ר�⣺
������ƻ�������ʽΪC4H6O5��0��l molƻ����������NaHCO3��Һ��Ӧ�ܲ���4.48L CO2����״������������̼�����ʵ���Ϊ0.2mol����1molƻ���Ậ2mol-COOH��ƻ������ˮ������ʹ��ˮ��ɫ�IJ��Ӧ����1��-OH�����ƻ����ķ���ʽ֪��ƻ����Ľṹ��ʽΪ��HOOCCH2CH��OH��COOH��ƻ����������Ӧ���еľۺ����ɾ�ƻ���ᣨPMLA������ṹΪ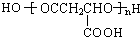 ��D����������E����E�к�����ԭ�ӣ�E���������Ƶ�ˮ��Һ������Ӧ����F��F�ữ����MLA������F�Ľṹ��ʽΪ��NaOOCCH2CH��OH��COONa��E�Ľṹ��ʽΪ��HOOCCH2CHBrCOOH��D�ܷ���������Ӧ��D�к���ȩ��������D�Ľṹ��ʽΪ��OHCCH2CHBrCHO������1��3-����ϩ��D�Ľṹ��ʽ֪��1��3-����ϩ���巢��1��4�ӳ�����AΪBrCH2CH=CHCH2Br��A���������Ƶ�ˮ��Һ����ȡ����Ӧ����BΪHOCH2CH=CHCH2OH��B��HBr�����ӳɷ�Ӧ����C��C�Ľṹ��ʽΪ��HOCH2CH2CHBrCH2OH��C�ٱ���������D��ƻ���ᾭ�ۺ����ɾ�ƻ���ᣨPMLA�����ݴ˴��⣮
��D����������E����E�к�����ԭ�ӣ�E���������Ƶ�ˮ��Һ������Ӧ����F��F�ữ����MLA������F�Ľṹ��ʽΪ��NaOOCCH2CH��OH��COONa��E�Ľṹ��ʽΪ��HOOCCH2CHBrCOOH��D�ܷ���������Ӧ��D�к���ȩ��������D�Ľṹ��ʽΪ��OHCCH2CHBrCHO������1��3-����ϩ��D�Ľṹ��ʽ֪��1��3-����ϩ���巢��1��4�ӳ�����AΪBrCH2CH=CHCH2Br��A���������Ƶ�ˮ��Һ����ȡ����Ӧ����BΪHOCH2CH=CHCH2OH��B��HBr�����ӳɷ�Ӧ����C��C�Ľṹ��ʽΪ��HOCH2CH2CHBrCH2OH��C�ٱ���������D��ƻ���ᾭ�ۺ����ɾ�ƻ���ᣨPMLA�����ݴ˴��⣮
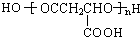 ��D����������E����E�к�����ԭ�ӣ�E���������Ƶ�ˮ��Һ������Ӧ����F��F�ữ����MLA������F�Ľṹ��ʽΪ��NaOOCCH2CH��OH��COONa��E�Ľṹ��ʽΪ��HOOCCH2CHBrCOOH��D�ܷ���������Ӧ��D�к���ȩ��������D�Ľṹ��ʽΪ��OHCCH2CHBrCHO������1��3-����ϩ��D�Ľṹ��ʽ֪��1��3-����ϩ���巢��1��4�ӳ�����AΪBrCH2CH=CHCH2Br��A���������Ƶ�ˮ��Һ����ȡ����Ӧ����BΪHOCH2CH=CHCH2OH��B��HBr�����ӳɷ�Ӧ����C��C�Ľṹ��ʽΪ��HOCH2CH2CHBrCH2OH��C�ٱ���������D��ƻ���ᾭ�ۺ����ɾ�ƻ���ᣨPMLA�����ݴ˴��⣮
��D����������E����E�к�����ԭ�ӣ�E���������Ƶ�ˮ��Һ������Ӧ����F��F�ữ����MLA������F�Ľṹ��ʽΪ��NaOOCCH2CH��OH��COONa��E�Ľṹ��ʽΪ��HOOCCH2CHBrCOOH��D�ܷ���������Ӧ��D�к���ȩ��������D�Ľṹ��ʽΪ��OHCCH2CHBrCHO������1��3-����ϩ��D�Ľṹ��ʽ֪��1��3-����ϩ���巢��1��4�ӳ�����AΪBrCH2CH=CHCH2Br��A���������Ƶ�ˮ��Һ����ȡ����Ӧ����BΪHOCH2CH=CHCH2OH��B��HBr�����ӳɷ�Ӧ����C��C�Ľṹ��ʽΪ��HOCH2CH2CHBrCH2OH��C�ٱ���������D��ƻ���ᾭ�ۺ����ɾ�ƻ���ᣨPMLA�����ݴ˴��⣮���
�⣺ƻ�������ʽΪC4H6O5��0��l molƻ����������NaHCO3��Һ��Ӧ�ܲ���4.48L CO2����״������������̼�����ʵ���Ϊ0.2mol����1molƻ���Ậ2mol-COOH��ƻ������ˮ������ʹ��ˮ��ɫ�IJ��Ӧ����1��-OH�����ƻ����ķ���ʽ֪��ƻ����Ľṹ��ʽΪ��HOOCCH2CH��OH��COOH��ƻ����������Ӧ���еľۺ����ɾ�ƻ���ᣨPMLA������ṹΪ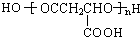 ��D����������E����E�к�����ԭ�ӣ�E���������Ƶ�ˮ��Һ������Ӧ����F��F�ữ����MLA������F�Ľṹ��ʽΪ��NaOOCCH2CH��OH��COONa��E�Ľṹ��ʽΪ��HOOCCH2CHBrCOOH��D�ܷ���������Ӧ��D�к���ȩ��������D�Ľṹ��ʽΪ��OHCCH2CHBrCHO������1��3-����ϩ��D�Ľṹ��ʽ֪��1��3-����ϩ���巢��1��4�ӳ�����AΪBrCH2CH=CHCH2Br��A���������Ƶ�ˮ��Һ����ȡ����Ӧ����BΪHOCH2CH=CHCH2OH��B��HBr�����ӳɷ�Ӧ����C��C�Ľṹ��ʽΪ��HOCH2CH2CHBrCH2OH��C�ٱ���������D��ƻ���ᾭ�ۺ����ɾ�ƻ���ᣨPMLA����
��D����������E����E�к�����ԭ�ӣ�E���������Ƶ�ˮ��Һ������Ӧ����F��F�ữ����MLA������F�Ľṹ��ʽΪ��NaOOCCH2CH��OH��COONa��E�Ľṹ��ʽΪ��HOOCCH2CHBrCOOH��D�ܷ���������Ӧ��D�к���ȩ��������D�Ľṹ��ʽΪ��OHCCH2CHBrCHO������1��3-����ϩ��D�Ľṹ��ʽ֪��1��3-����ϩ���巢��1��4�ӳ�����AΪBrCH2CH=CHCH2Br��A���������Ƶ�ˮ��Һ����ȡ����Ӧ����BΪHOCH2CH=CHCH2OH��B��HBr�����ӳɷ�Ӧ����C��C�Ľṹ��ʽΪ��HOCH2CH2CHBrCH2OH��C�ٱ���������D��ƻ���ᾭ�ۺ����ɾ�ƻ���ᣨPMLA����
��1��ͨ�����Ϸ���֪��B��D�Ľṹ��ʽ�ֱ�Ϊ��CH2OHCH=CHCH2OH��OHCCH2CHBrCHO��
�ʴ�Ϊ��CH2OHCH=CHCH2OH��OHCCH2CHBrCHO��
��2��AΪCH2BrCH=CHCH2Br��A�к��еĹ����ŵ�����Ϊ��ԭ�Ӻ�̼̼˫����ƻ����Ľṹ��ʽΪ��HOOCCH2CHOHCOOH��ƻ�����к���5����ԭ�ӣ����Ժ˴Ź���������5��壬
�ʴ�Ϊ����ԭ�Ӻ�̼̼˫����5��
��3����MLA������ͬ�����ŵ�ͬ���칹����HOOCC��CH3��HOHCOOH��HOOCCH��CH2OH��COOH�����Թ���2�֣�
�ʴ�Ϊ��2��
��4���ڼ��������£�E���������Ƶ�ˮ��Һ����ȡ����Ӧ����F������E��Fת���Ļ�ѧ����ʽΪ�� ���÷�ӦΪȡ����Ӧ��
���÷�ӦΪȡ����Ӧ��
�ʴ�Ϊ�� ��ȡ����Ӧ��
��ȡ����Ӧ��
��5��˳���ܵߵ�������������B��̼̼˫��Ҳ���������Ӷ��ﲻ��ʵ��Ŀ�ģ�
�ʴ�Ϊ�����ܣ�����������B��̼̼˫��Ҳ��������
��6��PMLA�������õ����������ԣ�������Ϊ��������ߵȲ���Ӧ��������ҽҩ�������������������������ˮ��Ļ�ѧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
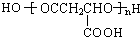 ��D����������E����E�к�����ԭ�ӣ�E���������Ƶ�ˮ��Һ������Ӧ����F��F�ữ����MLA������F�Ľṹ��ʽΪ��NaOOCCH2CH��OH��COONa��E�Ľṹ��ʽΪ��HOOCCH2CHBrCOOH��D�ܷ���������Ӧ��D�к���ȩ��������D�Ľṹ��ʽΪ��OHCCH2CHBrCHO������1��3-����ϩ��D�Ľṹ��ʽ֪��1��3-����ϩ���巢��1��4�ӳ�����AΪBrCH2CH=CHCH2Br��A���������Ƶ�ˮ��Һ����ȡ����Ӧ����BΪHOCH2CH=CHCH2OH��B��HBr�����ӳɷ�Ӧ����C��C�Ľṹ��ʽΪ��HOCH2CH2CHBrCH2OH��C�ٱ���������D��ƻ���ᾭ�ۺ����ɾ�ƻ���ᣨPMLA����
��D����������E����E�к�����ԭ�ӣ�E���������Ƶ�ˮ��Һ������Ӧ����F��F�ữ����MLA������F�Ľṹ��ʽΪ��NaOOCCH2CH��OH��COONa��E�Ľṹ��ʽΪ��HOOCCH2CHBrCOOH��D�ܷ���������Ӧ��D�к���ȩ��������D�Ľṹ��ʽΪ��OHCCH2CHBrCHO������1��3-����ϩ��D�Ľṹ��ʽ֪��1��3-����ϩ���巢��1��4�ӳ�����AΪBrCH2CH=CHCH2Br��A���������Ƶ�ˮ��Һ����ȡ����Ӧ����BΪHOCH2CH=CHCH2OH��B��HBr�����ӳɷ�Ӧ����C��C�Ľṹ��ʽΪ��HOCH2CH2CHBrCH2OH��C�ٱ���������D��ƻ���ᾭ�ۺ����ɾ�ƻ���ᣨPMLA������1��ͨ�����Ϸ���֪��B��D�Ľṹ��ʽ�ֱ�Ϊ��CH2OHCH=CHCH2OH��OHCCH2CHBrCHO��
�ʴ�Ϊ��CH2OHCH=CHCH2OH��OHCCH2CHBrCHO��
��2��AΪCH2BrCH=CHCH2Br��A�к��еĹ����ŵ�����Ϊ��ԭ�Ӻ�̼̼˫����ƻ����Ľṹ��ʽΪ��HOOCCH2CHOHCOOH��ƻ�����к���5����ԭ�ӣ����Ժ˴Ź���������5��壬
�ʴ�Ϊ����ԭ�Ӻ�̼̼˫����5��
��3����MLA������ͬ�����ŵ�ͬ���칹����HOOCC��CH3��HOHCOOH��HOOCCH��CH2OH��COOH�����Թ���2�֣�
�ʴ�Ϊ��2��
��4���ڼ��������£�E���������Ƶ�ˮ��Һ����ȡ����Ӧ����F������E��Fת���Ļ�ѧ����ʽΪ��
 ���÷�ӦΪȡ����Ӧ��
���÷�ӦΪȡ����Ӧ���ʴ�Ϊ��
 ��ȡ����Ӧ��
��ȡ����Ӧ����5��˳���ܵߵ�������������B��̼̼˫��Ҳ���������Ӷ��ﲻ��ʵ��Ŀ�ģ�
�ʴ�Ϊ�����ܣ�����������B��̼̼˫��Ҳ��������
��6��PMLA�������õ����������ԣ�������Ϊ��������ߵȲ���Ӧ��������ҽҩ�������������������������ˮ��Ļ�ѧ����ʽΪ��
 ��
���ʴ�Ϊ��
 ��
��
���������⿼���л�����ƶϣ���ȷƻ������������ƶ���ṹ�ǽ����Ĺؼ�����Ϥ�������ᡢϩ�������ʼ��ɽ��ͬ���칹��������ж���ѧϰ�ѵ㣬��Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
 M-3�����������ٴ�����ε�С���ӿ���ҩ���ṹ��ͼ��ʾ��δ��ʾ����ռ乹�ͣ������й���NM-3������������ȷ���ǣ�������
M-3�����������ٴ�����ε�С���ӿ���ҩ���ṹ��ͼ��ʾ��δ��ʾ����ռ乹�ͣ������й���NM-3������������ȷ���ǣ�������| A������������̼ԭ�� |
| B����ʹ������Ȼ�̼��Һ��ɫ |
| C������FeC13��Һ������ɫ��Ӧ |
| D��1mo1������������2mo1NaOH��Ӧ |
X��Y��Z��M��WΪ���ֶ�����Ԫ�أ����������ڱ������λ�����±���������˵����ȷ���ǣ�������
| M | |||||||
| X | Y | Z | |||||
| W |
| A��Y��M�γɵ���̬�������ڱ�״���µ��ܶ�Ϊ0.76 g��L-1 |
| B��ԭ�Ӱ뾶��W��Z��Y��X��M |
| C����XԪ���γɵĵ���һ����ԭ�Ӿ��� |
| D��XZ2��X2M2��W2Z2��Ϊֱ���͵Ĺ��ۻ����� |
��֪������1mol H-H����ʱ��������Ϊ436kJ����������1mol O=O����ȫ����ʱ��������496kJ��ˮ������1mol H-O���γ�ʱ�ų�����463kJ����1mol������ȫȼ������ˮ����ʱ�ų�������������
| A��221 kJ |
| B��557 kJ |
| C��242 kJ |
| D��188 kJ |
����˵����ȷ���ǣ�������
| A����AlCl3��Һ��Al2��SO4��3��Һ�ֱ���ȡ����ɡ����գ����ù���ɷ���ͬ |
| B������FeSO4��Һʱ����FeSO4��������ϡ�����У�Ȼ��ϡ��������Ũ�� |
| C����ľ�ҿ������̬���ʻ��ʹ�� |
| D���������ƣ�Na2FeO4����Ӧ��������ˮ����������ˮ |



 �ж���ͬ���칹�壬д��2�ֺ���1��ȩ����2���ǻ��ұ�����ֻ��2��һ��ȡ����ķ����廯����Ľṹ��ʽ��
�ж���ͬ���칹�壬д��2�ֺ���1��ȩ����2���ǻ��ұ�����ֻ��2��һ��ȡ����ķ����廯����Ľṹ��ʽ�� ��·��
��·�� �ڼ�����д����Ӧ�Լ�����Ӧ��������
�ڼ�����д����Ӧ�Լ�����Ӧ��������