��Ŀ����
������������ͭ����������������ϢϢ��أ�
��1���ۺ������������PFS���Ļ�ѧʽΪ[Fe��OH��n��SO4��
]n���dz��õ�ˮ����������PFS����Ԫ�ؼ�̬��ͬ�������ӵĵ����Ų�ʽΪ ��
��2�����й���[Cu��NH3��4]SO4��K4[Fe��CN��6]��Na3[AlF6]��˵������ȷ���� ������ĸ����
a�����������к��еĻ�ѧ�����;������Ӽ�����λ��
b��[Cu��NH3��4]SO4�к���NH3���ӣ���ˮ��Һ��Ҳ���д�������
c���������ʵ����Ԫ���е�һ�����������ǵ�Ԫ�ء�
d��K4[Fe��CN��6]��Na3[AlF6]���������Ӿ�����ͬ����λ��
��3���Ⱥͼ��벻ͬ��̬��ͭ���������ֻ�����������Ӿ�Ϊ�������ṹ����ͼ��ʾ����aλ���ϵ�Clԭ�ӵ��ӻ��������Ϊ ����֪����һ�ֻ�����Ļ�ѧʽΪKCuCl3������һ�ֻ�����Ļ�ѧʽΪ ��

��4���þ����X�������䷨���Բ�ð����ӵ�������ֵ���Խ���ͭ�IJⶨ�õ����½����ͭ����Ϊ�����������ܶѻ����߳�Ϊ361pm����֪ͭ���ܶ�Ϊ9 00g?cm-3����ͭԭ�ӵ�ֱ��ԼΪ pm�������ӵ�������ֵΪ ��
��1���ۺ������������PFS���Ļ�ѧʽΪ[Fe��OH��n��SO4��
| 3-n |
| 2 |
��2�����й���[Cu��NH3��4]SO4��K4[Fe��CN��6]��Na3[AlF6]��˵������ȷ����
a�����������к��еĻ�ѧ�����;������Ӽ�����λ��
b��[Cu��NH3��4]SO4�к���NH3���ӣ���ˮ��Һ��Ҳ���д�������
c���������ʵ����Ԫ���е�һ�����������ǵ�Ԫ�ء�
d��K4[Fe��CN��6]��Na3[AlF6]���������Ӿ�����ͬ����λ��
��3���Ⱥͼ��벻ͬ��̬��ͭ���������ֻ�����������Ӿ�Ϊ�������ṹ����ͼ��ʾ����aλ���ϵ�Clԭ�ӵ��ӻ��������Ϊ

��4���þ����X�������䷨���Բ�ð����ӵ�������ֵ���Խ���ͭ�IJⶨ�õ����½����ͭ����Ϊ�����������ܶѻ����߳�Ϊ361pm����֪ͭ���ܶ�Ϊ9 00g?cm-3����ͭԭ�ӵ�ֱ��ԼΪ
���㣺�����ļ���,��������Ԫ�صĵ��ʼ��仯������ۺ�Ӧ��
ר�⣺Ԫ�ؼ��仯����
��������1�����õ�ˮ����������֪������ˮ�����ɽ��壬��Ϊ+3������
��2��a������������������λ����֮������Ӽ�����λ�����к���λ����
b��������NH3���ӣ�
c���ǽ�������ǿ��ΪF����縺�����
d���ɻ�ѧʽ��֪����λ����Ϊ6��
��3����ͼ֪aλ���ϵ�Clԭ���γ���2���Ҽ���ͬʱ����2���µ��Ӷԣ����ӻ��������Ϊsp3�ӻ�������ͭ�Ļ��ϼ���+1��+2����֪KCuCl3��ͭΪ+2�ۣ���+1�۵�ͭ�γɻ�����Ļ�ѧʽΪK2CuCl3��
��4������ͭ����Ϊ�����������ܶѻ����ɱ߳��ɼ�������������������m=�ѡ�V�����������ɾ�����֪Cuԭ���ڶ�������ģ��Դ˼��㣮
��2��a������������������λ����֮������Ӽ�����λ�����к���λ����
b��������NH3���ӣ�
c���ǽ�������ǿ��ΪF����縺�����
d���ɻ�ѧʽ��֪����λ����Ϊ6��
��3����ͼ֪aλ���ϵ�Clԭ���γ���2���Ҽ���ͬʱ����2���µ��Ӷԣ����ӻ��������Ϊsp3�ӻ�������ͭ�Ļ��ϼ���+1��+2����֪KCuCl3��ͭΪ+2�ۣ���+1�۵�ͭ�γɻ�����Ļ�ѧʽΪK2CuCl3��
��4������ͭ����Ϊ�����������ܶѻ����ɱ߳��ɼ�������������������m=�ѡ�V�����������ɾ�����֪Cuԭ���ڶ�������ģ��Դ˼��㣮
���
�⣺��1�����õ�ˮ����������֪������ˮ�����ɽ��壬��Ϊ+3�������������ӵĵ����Ų�Ϊ1s22s22p63s23p63d5��[Ar]3d5��
�ʴ�Ϊ��1s22s22p63s23p63d5��[Ar]3d5��
��2��a�������д��ڻ�ѧ������Ϊ��λ�������Ӽ�����a��ȷ��
b��[Cu��NH3��4]SO4��ͭ���ӺͰ�����֮���γ���λ����������NH3���ӣ���ˮ��Һ��Ҳ�����д���NH3���ӣ���b����
c���������ʵ����Ԫ���е�һ�����������Ƿ�Ԫ�أ���c����
d��K4[Fe��CN��6]��Na3[AlF6]���������Ӿ�����ͬ����λ������Ϊ6����d��ȷ��
�ʴ�Ϊ��ad��
��3��aλ����Clԭ�ӳ�2������������2�Թ¶Ե��ӣ��ӻ������Ϊ4���ӻ��������Ϊ��sp3��
һ�ֻ�����Ļ�ѧʽΪKCuCl3������ͭԪ��Ϊ+2�ۣ�����һ�ֻ�������ͭΪ+1�ۣ�CuCl3ԭ���ŵĻ��ϼ�Ϊ-2���仯ѧʽΪK2CuCl3��
�ʴ�Ϊ��sp3��K2CuCl3��
��4������ͭ����Ϊ�����������ܶѻ����ɱ߳��ɼ�������������V=��361 pm��3��4.70��10-23 cm3������m=�ѡ�V=9.00 g?cm-3��4.70��10-23cm3=4.23��10-22g��
����һ��ͭ�����к��е�ͭԭ����Ϊ8��
+6��
=4��������ÿ��ͭԭ�ӵ����ԼΪ=4.70��10-23 cm3��4=1.18��10-23 cm3����
���С�d3=1.18��10-23 cm3�����ͭԭ�ӵ�ֱ��d��255pm��NA=
��6.05��1023mol-1��
�ʴ�Ϊ��255��6.05��1023��
�ʴ�Ϊ��1s22s22p63s23p63d5��[Ar]3d5��
��2��a�������д��ڻ�ѧ������Ϊ��λ�������Ӽ�����a��ȷ��
b��[Cu��NH3��4]SO4��ͭ���ӺͰ�����֮���γ���λ����������NH3���ӣ���ˮ��Һ��Ҳ�����д���NH3���ӣ���b����
c���������ʵ����Ԫ���е�һ�����������Ƿ�Ԫ�أ���c����
d��K4[Fe��CN��6]��Na3[AlF6]���������Ӿ�����ͬ����λ������Ϊ6����d��ȷ��
�ʴ�Ϊ��ad��
��3��aλ����Clԭ�ӳ�2������������2�Թ¶Ե��ӣ��ӻ������Ϊ4���ӻ��������Ϊ��sp3��
һ�ֻ�����Ļ�ѧʽΪKCuCl3������ͭԪ��Ϊ+2�ۣ�����һ�ֻ�������ͭΪ+1�ۣ�CuCl3ԭ���ŵĻ��ϼ�Ϊ-2���仯ѧʽΪK2CuCl3��
�ʴ�Ϊ��sp3��K2CuCl3��
��4������ͭ����Ϊ�����������ܶѻ����ɱ߳��ɼ�������������V=��361 pm��3��4.70��10-23 cm3������m=�ѡ�V=9.00 g?cm-3��4.70��10-23cm3=4.23��10-22g��
����һ��ͭ�����к��е�ͭԭ����Ϊ8��
| 1 |
| 8 |
| 1 |
| 2 |
| 1 |
| 6 |
| 63.6g/mol | ||
|
�ʴ�Ϊ��255��6.05��1023��
���������⿼�龧�����㼰���ʽṹ��Ϊ��Ƶ���㣬����ѡ�������ʽṹ�����ʵĿ��飬�漰�����Ų����ӻ�����������ȣ�ע�ظ�Ƶ����Ŀ��飬�ۺ��Խ�ǿ����Ŀ�ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
���ֶ�����Ԫ��W��X��Y��Zԭ����������������ԭ�ӵ�����������֮��Ϊ19��W��XԪ��ԭ����������֮��Ϊ1��2��X2+��Z-���ӵĵ�����֮��Ϊ8������˵����ȷ���ǣ�������
| A����W���ڵ�ͬ����Ԫ�ص��ʵ���Ҫ��;���������� |
| B��X���ʲ������û���W���� |
| C��Ԫ��ԭ�Ӱ뾶�Ӵ�С��˳����X��Y��Z |
| D���ɷǽ�����ǿ����֪����������W�ĺ������Ʊ�Z�ĺ����� |
һ���¶��£������������Ϊ2.0L�ĺ����ܱ������з������·�Ӧ��PCl5��g��?PCl3��g��+Cl2��g��
����˵����ȷ���ǣ�������
| ��� | �¶ȣ��棩 | ��ʼ���ʵ��� ��mol�� | ƽ�����ʵ��� ��mol�� | �ﵽƽ������ʱ�� ��s�� | |
| PCl5��g�� | PCl3��g�� | Cl2��g�� | |||
| �� | 320 | 0.40 | 0.10 | 0.10 | t1 |
| �� | 320 | 0.80 | t2 | ||
| �� | 410 | 0.40 | 0.15 | 0.15 | t3 |
| A��ƽ�ⳣ��K�������������� | ||
| B����Ӧ����ƽ��ʱ��PCl5��ת���ʣ������������� | ||
C����Ӧ����ƽ��ʱ������I�е�ƽ������Ϊv��PCl5��=
| ||
| D����ʼʱ���������г���PCl5 0.30 mol��PCl30.45 mol��Cl20.10 mol����Ӧ�����淴Ӧ������� |
��֪25��ʱ�ܽ�ȣ�AgCl��AgI������5mL����KCl��KI��Ϊ0.01mol/L����Һ�У�����8mL 0.01mol/L AgNO3��Һ����ʱ��Һ���������ʵ�����Ũ�ȴ�С��ϵ��ȷ���ǣ�������
| A��c��K+����c��NO3-����c��Ag+��=c��Cl-��+c��I-�� |
| B��c��K+����c��NO3-����c��Ag+����c��Cl-����c��I-�� |
| C��c��NO3-����c��K+����c��Ag+����c��Cl-����c��I-�� |
| D��c��K+����c��NO3-����c��Cl-����c��Ag+����c��I-�� |
����ͼʾ���Ӧ������������ǣ�������
A��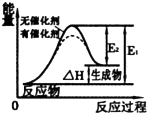 ͼ��ʾ�����ܸı仯ѧ��Ӧ���ʱ� |
B��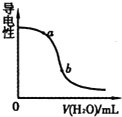 ͼ��ʾ��ˮ�м�ˮʱ��Һ�����Եı仯���������Һc��OH-����С��a��b |
C��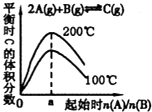 ��ͼ��֪��Ӧ2A��g��+B��g��?C��g���ġ�H��O���� a=2 |
D��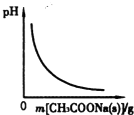 ͼ��ʾ��CH3COOH��Һ������CH3COONa�������ҺpH�ı仯��� |
 �����£���1L�� 0.1mol?L-1��HX��Һ��0.1mol?L-1ijһԪ��ROH��Һ��ˮϡ�ͣ�����ҺpH���ˮ����仯������ͼ������˵����ȷ���ǣ�������
�����£���1L�� 0.1mol?L-1��HX��Һ��0.1mol?L-1ijһԪ��ROH��Һ��ˮϡ�ͣ�����ҺpH���ˮ����仯������ͼ������˵����ȷ���ǣ�������| A��HXΪһ�����ᣬROHΪ���� |
| B��a��b������ˮ�����c��H+����Ϊ10-12mol?L-1 |
| C������Һ�������ϣ�c��X-��=c��R+��+c��ROH�� |
| D������Һ�������ϣ�c��R+����c��X-����c��OH-����c��H+�� |
��ý�屨����һЩ�Ƿ��ͳ�����ͩ�͡��������͵������㾫���ҳɻ����ͣ���ͼ�ṹ����һ���㾫�� �����жԸ��㾫����������ȷ���ǣ�������
�����жԸ��㾫����������ȷ���ǣ�������
 �����жԸ��㾫����������ȷ���ǣ�������
�����жԸ��㾫����������ȷ���ǣ�������| A�����㾫������������ˮ���ǿ��淴Ӧ |
| B�����㾫�ܹ�ʹ����KMnO4��Һ��ɫ |
| C�����㾫����������Ȼ�̼�����ӳɷ�Ӧ |
| D��һ�������£�1mol���㾫��H2�������ӳɷ�Ӧ�������Ҫ5molH 2 |
���Ӽ����ۼ�����ͬʱ������ͬһ�������У����л������мȴ������Ӽ��ִ��ڹ��ۼ����ǣ�������
| A��KBr |
| B��NaOH |
| C��HBr |
| D��NH4NO3 |
