��Ŀ����
ʵ����������0.5mol/L��NaOH��Һ500mL��
��1��������Һʱ��һ����Է�Ϊ���¼������裺
�ٳ��� �ڼ��� ���ܽ� ��ҡ�� ��ת�� ��ϴ�� �߶��� ����ȴ
����ȷ�IJ���˳��Ϊ ��
��2����ʵ���õ������������ձ�����ƽ�����롢���ӣ���ҩ�ף���ȱ�ٵ������ǣ� �� �� ��
��3��ijͬѧ������NaOH��������������������ƽ�����ձ�����������ƽƽ����״̬��ͼ����ͼ���п��Կ�������ͬѧ�ڲ���ʱ��һ�������� ���ձ���ʵ������Ϊ g��

��4��ʹ������ƿǰ������е�һ�������� ��
��5�������ƹ����У����в������������ƫ�ߵ��� ����д��ţ���
��û��ϴ���ձ��Ͳ�����
��ת����Һʱ������������������ƿ����
������ƿ�����������������ˮ
�ܶ���ʱijͬѧ�۲�Һ��������ͼ����ʾ������ƫ�ߣ�
�ݳ����������ƹ��壬��ʱ̫��
���ݺ�����ƿ������ҡ�ȣ����ú�Һ����ڿ̶��ߣ��ټ�ˮ���̶��ߣ�
��1��������Һʱ��һ����Է�Ϊ���¼������裺
�ٳ��� �ڼ��� ���ܽ� ��ҡ�� ��ת�� ��ϴ�� �߶��� ����ȴ
����ȷ�IJ���˳��Ϊ
��2����ʵ���õ������������ձ�����ƽ�����롢���ӣ���ҩ�ף���ȱ�ٵ������ǣ�
��3��ijͬѧ������NaOH��������������������ƽ�����ձ�����������ƽƽ����״̬��ͼ����ͼ���п��Կ�������ͬѧ�ڲ���ʱ��һ��������

��4��ʹ������ƿǰ������е�һ��������
��5�������ƹ����У����в������������ƫ�ߵ���
��û��ϴ���ձ��Ͳ�����
��ת����Һʱ������������������ƿ����
������ƿ�����������������ˮ
�ܶ���ʱijͬѧ�۲�Һ��������ͼ����ʾ������ƫ�ߣ�
�ݳ����������ƹ��壬��ʱ̫��
���ݺ�����ƿ������ҡ�ȣ����ú�Һ����ڿ̶��ߣ��ټ�ˮ���̶��ߣ�
���㣺����һ�����ʵ���Ũ�ȵ���Һ
ר�⣺��ѧʵ���������
��������1����������Һ��ʵ��������̽���ʵ�鲽������
��2�����ݲ��������и�����������ѡȡ������
��3��ʹ����ƽ����ҩƷʱҪ��ѭ��������������ԭ����������ҩƷλ�õߵ�����ʹ�������룬ʵ��ҩƷ������=���������-�����������
��4������ƿʹ��֮ǰӦ�������Ƿ�©ˮ��
��5������c=
�ж�������Һ��Ũ���Ƿ�ƫ���ƫС�����nƫ���vƫС��������Һ��Ũ��ƫ�ߣ����nƫС��vƫ��������Һ��Ũ�Ⱦ�ƫ�ͣ�
��2�����ݲ��������и�����������ѡȡ������
��3��ʹ����ƽ����ҩƷʱҪ��ѭ��������������ԭ����������ҩƷλ�õߵ�����ʹ�������룬ʵ��ҩƷ������=���������-�����������
��4������ƿʹ��֮ǰӦ�������Ƿ�©ˮ��
��5������c=
| n |
| V |
���
�⣺��1������0.5mol?L-1��NaOH��Һ500mL�����Ʋ����м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ���������ƽ����NaOH���õ�ҩ�ף������ձ���ϡ�ͣ����ò��������裬�����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ��2-3�Σ�����ϴ��Һ��������ƿ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ����Բ���˳���Ǣڢ٢ۢ�ݢޢߢܣ��ʴ�Ϊ���ڢ٢ۢ�ݢޢߢܣ�
��2������0.5mol?L-1��NaOH��Һ500mL�����Ʋ����г������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ���������ƽ����NaOH���õ�ҩ�ף������ձ���ϡ�ͣ����ò��������裬�����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ��2-3�Σ�����ϴ��Һ��������ƿ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�
�ʴ�Ϊ��500mL����ƿ������������ͷ�ιܣ�
��3�����ݡ�������������ԭ��֪������������̣��ձ��������̣����������Ʒ��λ�÷ŷ��ˣ���ҩƷ������=30g-2.6g=27.4g��
�ʴ�Ϊ������������̣��ձ��������̣����������Ʒ��λ�÷ŷ��ˣ���27.4��
��4������ƿʹ��֮ǰӦ�������Ƿ�©ˮ���ʴ�Ϊ����©��
��5������c=
�ж��Ƿ������
��û��ϴ���ձ��Ͳ��������������ʵ����ʵ���ƫ�ͣ�����������Һ��Ũ��ƫ�ͣ��ʴ���
��ת����Һʱ������������������ƿ���棬�������ʵ����ʵ���ƫ�ͣ�����������Һ��Ũ��ƫ�ͣ��ʴ���
������ƿ�����������������ˮ�����ʵ����ʵ�������Һ����������ı䣬����������Һ��Ũ����Ӱ�죬�ʴ���
�ܶ���ʱijͬѧ�۲�Һ��������ͼ����ʾ������ƫ�ߣ���������Һ�����ƫС��������Һ��Ũ��ƫ�ߣ�����ȷ��
�ݳ����������ƹ��壬��ʱ̫���������������Ƶ����ʵ���ƫ�ͣ�����������Һ��Ũ��ƫ�ͣ��ʴ���
���ݺ�����ƿ������ҡ�ȣ����ú�Һ����ڿ̶��ߣ��ټ�ˮ���̶��ߣ�������Һ�����ƫ��������Һ��Ũ��ƫ�ͣ��ʴ���
��ѡ�ܣ�
��2������0.5mol?L-1��NaOH��Һ500mL�����Ʋ����г������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ���������ƽ����NaOH���õ�ҩ�ף������ձ���ϡ�ͣ����ò��������裬�����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ��2-3�Σ�����ϴ��Һ��������ƿ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�
�ʴ�Ϊ��500mL����ƿ������������ͷ�ιܣ�
��3�����ݡ�������������ԭ��֪������������̣��ձ��������̣����������Ʒ��λ�÷ŷ��ˣ���ҩƷ������=30g-2.6g=27.4g��
�ʴ�Ϊ������������̣��ձ��������̣����������Ʒ��λ�÷ŷ��ˣ���27.4��
��4������ƿʹ��֮ǰӦ�������Ƿ�©ˮ���ʴ�Ϊ����©��
��5������c=
| n |
| V |
��û��ϴ���ձ��Ͳ��������������ʵ����ʵ���ƫ�ͣ�����������Һ��Ũ��ƫ�ͣ��ʴ���
��ת����Һʱ������������������ƿ���棬�������ʵ����ʵ���ƫ�ͣ�����������Һ��Ũ��ƫ�ͣ��ʴ���
������ƿ�����������������ˮ�����ʵ����ʵ�������Һ����������ı䣬����������Һ��Ũ����Ӱ�죬�ʴ���
�ܶ���ʱijͬѧ�۲�Һ��������ͼ����ʾ������ƫ�ߣ���������Һ�����ƫС��������Һ��Ũ��ƫ�ߣ�����ȷ��
�ݳ����������ƹ��壬��ʱ̫���������������Ƶ����ʵ���ƫ�ͣ�����������Һ��Ũ��ƫ�ͣ��ʴ���
���ݺ�����ƿ������ҡ�ȣ����ú�Һ����ڿ̶��ߣ��ټ�ˮ���̶��ߣ�������Һ�����ƫ��������Һ��Ũ��ƫ�ͣ��ʴ���
��ѡ�ܣ�
���������⿼����һ�����ʵ���Ũ����Һ�����ƣ�ע���c=
��������ԭ����ע���������Ƶij���Ӧ����С�ձ����ƽ��ʹ�ù����У���������ҩƷ�ŵߵ����������û��ʹ�ã���ҩƷ�������������ȣ����ʹ�����룬��ҩƷ������=����-���룮
| n |
| V |

��ϰ��ϵ�д�
�����Ŀ
����������S�Ļ��ϼ�Ϊ+4���ǣ�������
| A��S |
| B��SO2 |
| C��BaSO4 |
| D��H2SO4 |
��ʫ���ǹ���Ϊ�������µı�����Ƹ�������ʫ����ֻ�漰�����仯���ǣ�������
| A��Ұ���ղ��������紵���� |
| B�����ϵ���˿����������ɻ���ʼ�� |
| C��ֻҪ���������ĥ���� |
| D������һ�������꣬������ů������ |
���й��ڵ������Һ��˵������ȷ���ǣ�������
| A����0.1 mol/LCH3COONa��Һ��c(OH-)=c(CH3COOH)+c(H+) |
| B����0.1 mol/L��ˮ�м�����������粒��壬��ҺpH��С |
| C����ˮ�еμ��ռ���Һ����c��Cl-��+c��ClO-��=c��Na+��ʱ����Һһ���dz����� |
| D��ij�¶��½�ϡ��ˮ�μӵ�ϡ�����У�����Һ��pH=7ʱ��һ����c(Cl-)=c(NH4+) |
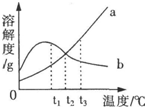 �й�a��b�������ʵ��ܽ��������ͼ��ʾ��������������ȷ���ǣ�������
�й�a��b�������ʵ��ܽ��������ͼ��ʾ��������������ȷ���ǣ�������| A��a���ʵ��ܽ�������¶ȵ����߶����� |
| B����t2��ʱ��a��b�������ʵ���Һ�����ʵ���������һ����� |
| C��t3��ʱ��a���ʵ��ܽ�ȴ���b���ʵ��ܽ�� |
| D����a��b�������ʵı�����Һ��t3�潵����t1�棬a�о���������b�������� |
��NA��ʾ�����ӵ�����������˵����ȷ���ǣ�������
| A��6.5g����п���п����ʱʧȥ�ĵ�����ĿΪ0.1NA |
| B�����³�ѹ�£�2g��������ԭ����ĿΪ2NA |
| C����״���£�11.2LH2O���еķ�����Ϊ0.5NA |
| D�����³�ѹ�£�11.2LCl2���еķ�����Ϊ0.5NA |
 ȡһ�����ʵ���Ũ�ȵ�NaOH��Һ100mL��Ȼ������ͨ��һ������CO2���壬�õ���ҺA����A����λ�������0.1mol/L��HCl��Һ��������CO2�����������״����������HCl��Һ�����֮���ϵ��ͼ��ʾ��ͨ������ش�
ȡһ�����ʵ���Ũ�ȵ�NaOH��Һ100mL��Ȼ������ͨ��һ������CO2���壬�õ���ҺA����A����λ�������0.1mol/L��HCl��Һ��������CO2�����������״����������HCl��Һ�����֮���ϵ��ͼ��ʾ��ͨ������ش�