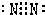��Ŀ����
����ΪԪ�����ڱ��е�һ���֣���ע�����������û�ѧ����ش��������⣺
��1��8��Ԫ���У���ѧ��������õ���______���ǽ�������ǿ����______��
��2���٢ڢݵ�����������Ӧˮ����ļ�����ǿ������˳��Ϊ______��
��3���ڢۢ����γɵļ����Ӱ뾶�ɴ�С��˳��Ϊ______��
��4�����⻯�ﳣ������Ԫ�آڵĹ������ﷴӦ�����ӷ���ʽΪ______��
��5���ٺ͢��γɵĵ���ɫ����ĵ���ʽΪ______��������ѧ��������Ϊ������Ӽ������ۼ�����______��
��6���ٺ͢ݵ�����������Ӧˮ�������Ӧ�����ӷ���ʽΪ______��
| ������ | ��A | ��A | ��A | ��A | ��A | ��A | ��A |
0 |
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� | ||||
| 4 | �� | �� |
��2���٢ڢݵ�����������Ӧˮ����ļ�����ǿ������˳��Ϊ______��
��3���ڢۢ����γɵļ����Ӱ뾶�ɴ�С��˳��Ϊ______��
��4�����⻯�ﳣ������Ԫ�آڵĹ������ﷴӦ�����ӷ���ʽΪ______��
��5���ٺ͢��γɵĵ���ɫ����ĵ���ʽΪ______��������ѧ��������Ϊ������Ӽ������ۼ�����______��
��6���ٺ͢ݵ�����������Ӧˮ�������Ӧ�����ӷ���ʽΪ______��
��1����ѧ��������õ�ӦΪ0��Ԫ�ض�Ӧ�ĵ��ʣ�ΪArԪ�أ������ڱ��У�ͬ���ڴ����ҷǽ���������ǿ��ͬ������ϵ��·ǽ�������������8��Ԫ���У�
�ǽ�������ǿ����F���ʴ�Ϊ��Ar��F��
��2���٢ڢݵ�����������Ӧˮ����ֱ���KOH��NaOH��Al��OH��3��Ԫ�صĽ�����Խǿ����Ӧ������������ˮ����ļ���Խǿ�����ݽ����Եĵݱ���ɿ�֪��
�����ԣ�K��Na��Al�������KOH��NaOH��Al��OH��3���ʴ�Ϊ��KOH��NaOH��Al��OH��3��
��3���ڢۢ����γɵļ����ӷֱ�ΪK+��Ca2+��Mg2+����ͬ���ڽ������Ӱ뾶��������С��ͬ�������Ӱ뾶���ϵ����������֪�����Ӱ뾶K+��Ca2+��Mg2+��
�ʴ�Ϊ��K+��Ca2+��Mg2+��
��4�����⻯��ΪH2O��Ԫ�آڵĹ�������ΪNa2O2�����߷���������ԭ��Ӧ����NaOH��O2��
��Ӧ�����ӷ���ʽΪ2H2O+2Na2O2=4Na++4OH-+O2�����ʴ�Ϊ��2H2O+2Na2O2=4Na++4OH-+O2����
��5���ٺ͢��γɵĵ���ɫ����Na2O2��Ϊ���ӻ��������ʽΪ

���������к������Ӽ����ۼ���
�ʴ�Ϊ��

�����Ӽ����ۼ���
��6���ٺ͢ݵ�����������Ӧˮ����ֱ�ΪNaOH��Al��OH��3��Al��OH��3�������ԣ�����NaOH��Ӧ����AlO2-��ˮ����Ӧ�����ӷ���ʽΪOH-+Al��OH��3 =AlO2-+2H2O��
�ʴ�Ϊ��OH-+Al��OH��3 =AlO2-+2H2O��
�ǽ�������ǿ����F���ʴ�Ϊ��Ar��F��
��2���٢ڢݵ�����������Ӧˮ����ֱ���KOH��NaOH��Al��OH��3��Ԫ�صĽ�����Խǿ����Ӧ������������ˮ����ļ���Խǿ�����ݽ����Եĵݱ���ɿ�֪��
�����ԣ�K��Na��Al�������KOH��NaOH��Al��OH��3���ʴ�Ϊ��KOH��NaOH��Al��OH��3��
��3���ڢۢ����γɵļ����ӷֱ�ΪK+��Ca2+��Mg2+����ͬ���ڽ������Ӱ뾶��������С��ͬ�������Ӱ뾶���ϵ����������֪�����Ӱ뾶K+��Ca2+��Mg2+��
�ʴ�Ϊ��K+��Ca2+��Mg2+��
��4�����⻯��ΪH2O��Ԫ�آڵĹ�������ΪNa2O2�����߷���������ԭ��Ӧ����NaOH��O2��
��Ӧ�����ӷ���ʽΪ2H2O+2Na2O2=4Na++4OH-+O2�����ʴ�Ϊ��2H2O+2Na2O2=4Na++4OH-+O2����
��5���ٺ͢��γɵĵ���ɫ����Na2O2��Ϊ���ӻ��������ʽΪ

���������к������Ӽ����ۼ���
�ʴ�Ϊ��

�����Ӽ����ۼ���
��6���ٺ͢ݵ�����������Ӧˮ����ֱ�ΪNaOH��Al��OH��3��Al��OH��3�������ԣ�����NaOH��Ӧ����AlO2-��ˮ����Ӧ�����ӷ���ʽΪOH-+Al��OH��3 =AlO2-+2H2O��
�ʴ�Ϊ��OH-+Al��OH��3 =AlO2-+2H2O��

��ϰ��ϵ�д�
 ����5��2���ϵ�д�
����5��2���ϵ�д�
�����Ŀ