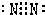��Ŀ����
����ΪԪ�����ڱ��е�һ���֣��û�ѧʽ��Ԫ�ط��Żش��������⣺
��1���٢ڢۢܵ�����������Ӧˮ���������ǿ������˳��Ϊ
��2���٢ۢߢ��γɵļ����Ӱ뾶�ɴ�С��˳��Ϊ
��3���ߢ��γɵ��⻯��е�ϸߵ���
��4������ ��Ԫ�غ˵��֮����
��Ԫ�غ˵��֮����
��5���ݵ�һ���⻯��A��Է�������Ϊ16����A��ˮ���ᄃ���У�ƽ��ÿ46��ˮ���ӹ���8����������ÿ��������������1��A���ӻ�ˮ���ӣ�����8������������6�����ɵ���A���ӣ�����2������ˮ������䣬����ˮ�����ƽ����ɿɱ�ʾΪ
| �� ���� |
IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
| �� | �� | �� | �� | �� | ||||
| �� | �� | �� | �� | �� | �� | |||
| �� | �� |  |
KOH��NaOH��Mg��OH��2��Al��OH��3
KOH��NaOH��Mg��OH��2��Al��OH��3
����2���٢ۢߢ��γɵļ����Ӱ뾶�ɴ�С��˳��Ϊ
O2-��F-��Na+��Mg2+
O2-��F-��Na+��Mg2+
����3���ߢ��γɵ��⻯��е�ϸߵ���
H20
H20
��ԭ�������Ӽ������
���Ӽ������
����4������
 ��Ԫ�غ˵��֮����
��Ԫ�غ˵��֮����18
18
����5���ݵ�һ���⻯��A��Է�������Ϊ16����A��ˮ���ᄃ���У�ƽ��ÿ46��ˮ���ӹ���8����������ÿ��������������1��A���ӻ�ˮ���ӣ�����8������������6�����ɵ���A���ӣ�����2������ˮ������䣬����ˮ�����ƽ����ɿɱ�ʾΪ
CH4?8H2O
CH4?8H2O
����������1������Ԫ�������ɣ�ͬ����Ԫ�ص�ԭ�Ӵ����Ҽ���������ͬ������ϵ��¼�������ǿ���ش�
��2����������Ų�һ�����˵����Խ��뾶ԽС��
��3��ˮ���ӽ���������·е�ϸߣ�
��4��Ԫ�غ˵��֮��=ԭ������֮�����ͬ����Ԫ�ص�ԭ�����������ش�
��5���������й�����Ȼ��ˮ���ﹹ�ɵ�������������ÿ8��������6��������CH4���ӣ�����2����2�������H2O��������䣬�ƶϸ���Ȼ��ˮ����Ĺ�����CH4������H2O���ӵ��������ıȣ��ж���Ȼ��ˮ�����ƽ����ɵı�ʾʽ��
��2����������Ų�һ�����˵����Խ��뾶ԽС��
��3��ˮ���ӽ���������·е�ϸߣ�
��4��Ԫ�غ˵��֮��=ԭ������֮�����ͬ����Ԫ�ص�ԭ�����������ش�
��5���������й�����Ȼ��ˮ���ﹹ�ɵ�������������ÿ8��������6��������CH4���ӣ�����2����2�������H2O��������䣬�ƶϸ���Ȼ��ˮ����Ĺ�����CH4������H2O���ӵ��������ıȣ��ж���Ȼ��ˮ�����ƽ����ɵı�ʾʽ��
����⣺����Ԫ�������ڱ��е�λ�ã�����ȷ������Na������K������Mg������Al������C������N������O������S������F������Cl�����һ����Br��
��1���٢ڢۢܵ�����������Ӧˮ����ֱ��ǣ��������ơ��������ء�������þ������������ͬ����Ԫ�ص�ԭ�Ӵ����Ҽ���������ͬ������ϵ��¼�������ǿ�����Լ���ǿ������˳��Ϊ��KOH��NaOH��Mg��OH��2��Al��OH��3���ʴ�Ϊ��KOH��NaOH��Mg��OH��2��Al��OH��3��
��2�������ӡ������ӡ�þ���Ӻͷ����ӵĺ�������Ų�һ�����˵����Խ��뾶ԽС���ʴ�Ϊ��O2-��F-��Na+��Mg2+��
��3�������Ԫ���γɵ��⻯��ֱ��������ˮ������ˮ���Ӽ���������·е�ϸߣ��ʴ�Ϊ��H20�����Ӽ��������
��4���˵��������ԭ��������Cl��Br��ԭ������֮��Ϊ18���ʴ�Ϊ��18��
��5��C���⻯������Է�������Ϊ16���Ǽ��飬��Ϊƽ��ÿ46��ˮ���ӹ���8������������ÿ8��������6��������CH4���ӣ�����2����2�������H2O��������䣬������Ȼ��ˮ����Ĺ����к�6��CH4���ӡ�46+2=48��H2O���ӣ���CH4������H2O������������=6��48=1��8�����ж���Ȼ��ˮ�����ƽ����ɿɱ�ʾΪCH4?8H2O���ʴ�Ϊ��CH4?8H2O��
��1���٢ڢۢܵ�����������Ӧˮ����ֱ��ǣ��������ơ��������ء�������þ������������ͬ����Ԫ�ص�ԭ�Ӵ����Ҽ���������ͬ������ϵ��¼�������ǿ�����Լ���ǿ������˳��Ϊ��KOH��NaOH��Mg��OH��2��Al��OH��3���ʴ�Ϊ��KOH��NaOH��Mg��OH��2��Al��OH��3��
��2�������ӡ������ӡ�þ���Ӻͷ����ӵĺ�������Ų�һ�����˵����Խ��뾶ԽС���ʴ�Ϊ��O2-��F-��Na+��Mg2+��
��3�������Ԫ���γɵ��⻯��ֱ��������ˮ������ˮ���Ӽ���������·е�ϸߣ��ʴ�Ϊ��H20�����Ӽ��������
��4���˵��������ԭ��������Cl��Br��ԭ������֮��Ϊ18���ʴ�Ϊ��18��
��5��C���⻯������Է�������Ϊ16���Ǽ��飬��Ϊƽ��ÿ46��ˮ���ӹ���8������������ÿ8��������6��������CH4���ӣ�����2����2�������H2O��������䣬������Ȼ��ˮ����Ĺ����к�6��CH4���ӡ�46+2=48��H2O���ӣ���CH4������H2O������������=6��48=1��8�����ж���Ȼ��ˮ�����ƽ����ɿɱ�ʾΪCH4?8H2O���ʴ�Ϊ��CH4?8H2O��
���������⿼��ѧ��Ԫ�����ڱ��Ľṹ�Լ�Ԫ�������ɵ����֪ʶ�����Ը�����ѧ֪ʶ���лش��ۺ��Խ�ǿ���ѶȽϴ�

��ϰ��ϵ�д�
 ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
�����Ŀ