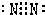��Ŀ����
����ΪԪ�����ڱ��е�һ���֣���ע�����������û�ѧ����ش��������⣺
��1��8��Ԫ���У���ѧ��������õ���
��2���٢ڢݵ�����������Ӧˮ����ļ�����ǿ������˳��Ϊ
��3���ڢۢ����γɵļ����Ӱ뾶�ɴ�С��˳��Ϊ
��4�����⻯�ﳣ������Ԫ�آڵĹ������ﷴӦ�����ӷ���ʽΪ
��5���ٺ͢��γɵĵ���ɫ����ĵ���ʽΪ
 ��������ѧ��������Ϊ������Ӽ������ۼ�����
��������ѧ��������Ϊ������Ӽ������ۼ�����
��6���ٺ͢ݵ�����������Ӧˮ�������Ӧ�����ӷ���ʽΪ
| ������ | ��A | ��A | ��A | ��A | ��A | ��A | ��A |
0 |
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� | ||||
| 4 | �� | �� |
Ar
Ar
���ǽ�������ǿ����F
F
����2���٢ڢݵ�����������Ӧˮ����ļ�����ǿ������˳��Ϊ
KOH��NaOH��Al��OH��3
KOH��NaOH��Al��OH��3
����3���ڢۢ����γɵļ����Ӱ뾶�ɴ�С��˳��Ϊ
K+��Ca2+��Mg2+
K+��Ca2+��Mg2+
����4�����⻯�ﳣ������Ԫ�آڵĹ������ﷴӦ�����ӷ���ʽΪ
2H2O+2Na2O2=4Na++4OH-+O2��
2H2O+2Na2O2=4Na++4OH-+O2��
����5���ٺ͢��γɵĵ���ɫ����ĵ���ʽΪ


���Ӽ����ۼ�
���Ӽ����ۼ�
����6���ٺ͢ݵ�����������Ӧˮ�������Ӧ�����ӷ���ʽΪ
OH-+Al��OH��3 =AlO2-+2H2O
OH-+Al��OH��3 =AlO2-+2H2O
������������Ԫ�������ڱ��е�λ�ÿ�֪Ԫ�����࣬����Ԫ�������ɵĵݱ���ɱȽϷǽ����ԡ����Ӱ뾶�Լ����ʵĽṹ�����ʣ�
����⣺��1����ѧ��������õ�ӦΪ0��Ԫ�ض�Ӧ�ĵ��ʣ�ΪArԪ�أ������ڱ��У�ͬ���ڴ����ҷǽ���������ǿ��ͬ������ϵ��·ǽ�������������8��Ԫ���У�
�ǽ�������ǿ����F���ʴ�Ϊ��Ar��F��
��2���٢ڢݵ�����������Ӧˮ����ֱ���KOH��NaOH��Al��OH��3��Ԫ�صĽ�����Խǿ����Ӧ������������ˮ����ļ���Խǿ�����ݽ����Եĵݱ���ɿ�֪��
�����ԣ�K��Na��Al�������KOH��NaOH��Al��OH��3���ʴ�Ϊ��KOH��NaOH��Al��OH��3��
��3���ڢۢ����γɵļ����ӷֱ�ΪK+��Ca2+��Mg2+����ͬ���ڽ������Ӱ뾶��������С��ͬ�������Ӱ뾶���ϵ����������֪�����Ӱ뾶K+��Ca2+��Mg2+��
�ʴ�Ϊ��K+��Ca2+��Mg2+��
��4�����⻯��ΪH2O��Ԫ�آڵĹ�������ΪNa2O2�����߷���������ԭ��Ӧ����NaOH��O2��
��Ӧ�����ӷ���ʽΪ2H2O+2Na2O2=4Na++4OH-+O2�����ʴ�Ϊ��2H2O+2Na2O2=4Na++4OH-+O2����
��5���ٺ͢��γɵĵ���ɫ����Na2O2��Ϊ���ӻ��������ʽΪ ���������к������Ӽ����ۼ���
���������к������Ӽ����ۼ���
�ʴ�Ϊ�� �����Ӽ����ۼ���
�����Ӽ����ۼ���
��6���ٺ͢ݵ�����������Ӧˮ����ֱ�ΪNaOH��Al��OH��3��Al��OH��3�������ԣ�����NaOH��Ӧ����AlO2-��ˮ����Ӧ�����ӷ���ʽΪOH-+Al��OH��3 =AlO2-+2H2O��
�ʴ�Ϊ��OH-+Al��OH��3 =AlO2-+2H2O��
�ǽ�������ǿ����F���ʴ�Ϊ��Ar��F��
��2���٢ڢݵ�����������Ӧˮ����ֱ���KOH��NaOH��Al��OH��3��Ԫ�صĽ�����Խǿ����Ӧ������������ˮ����ļ���Խǿ�����ݽ����Եĵݱ���ɿ�֪��
�����ԣ�K��Na��Al�������KOH��NaOH��Al��OH��3���ʴ�Ϊ��KOH��NaOH��Al��OH��3��
��3���ڢۢ����γɵļ����ӷֱ�ΪK+��Ca2+��Mg2+����ͬ���ڽ������Ӱ뾶��������С��ͬ�������Ӱ뾶���ϵ����������֪�����Ӱ뾶K+��Ca2+��Mg2+��
�ʴ�Ϊ��K+��Ca2+��Mg2+��
��4�����⻯��ΪH2O��Ԫ�آڵĹ�������ΪNa2O2�����߷���������ԭ��Ӧ����NaOH��O2��
��Ӧ�����ӷ���ʽΪ2H2O+2Na2O2=4Na++4OH-+O2�����ʴ�Ϊ��2H2O+2Na2O2=4Na++4OH-+O2����
��5���ٺ͢��γɵĵ���ɫ����Na2O2��Ϊ���ӻ��������ʽΪ
 ���������к������Ӽ����ۼ���
���������к������Ӽ����ۼ����ʴ�Ϊ��
 �����Ӽ����ۼ���
�����Ӽ����ۼ�����6���ٺ͢ݵ�����������Ӧˮ����ֱ�ΪNaOH��Al��OH��3��Al��OH��3�������ԣ�����NaOH��Ӧ����AlO2-��ˮ����Ӧ�����ӷ���ʽΪOH-+Al��OH��3 =AlO2-+2H2O��
�ʴ�Ϊ��OH-+Al��OH��3 =AlO2-+2H2O��
���������⿼��λ�ýṹ���ʵĹ�ϵ��Ӧ�ã���Ŀ�Ѷ��еȣ�ע�����Ԫ���������ڱ��е�λ�ý��Ԫ��������֪ʶ���

��ϰ��ϵ�д�
 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д� ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�
�����Ŀ