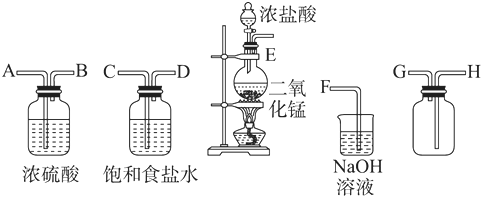
��Ŀ����
11��2Zn��OH��2•ZnCO3���Ʊ�����ZnO���м��壬��п��ɰ����Ҫ�ɷ�ΪZnO��������Cu2+��Mn2+�����ӣ�Ϊԭ���Ʊ�2Zn��OH��2•ZnCO3�Ĺ���������ͼ��
��ش��������⣺
��1������NH4��2SO4��NH3•H2O�Ļ����Һ�д���c��NH4+��=2c��SO42-��ʱ����Һ���У���ᡱ��������С����ԣ�
��2������ȡ��ʱΪ�����п�Ľ����ʣ��ɲ�ȡ�Ĵ�ʩ�ǽ��衢�ʵ����ȡ��ӳ���ȡʱ�䡢��ν�ȡ�ȣ���д���֣���
��3������ȡ��ʱ�����NH3•H2O����������MnO2�����ӷ���ʽΪMn2++H2O2+2NH3•H2O=MnO2��+2NH4++2H2O��
��4������S2-�ܽ�Cu2+�����������ȥ����ѡ��ZnS���г��ӣ��Ƿ���У��ü���˵��ԭ���У�ZnS+Cu2+?CuS+Zn2+��K=$\frac{Ksp��ZnS��}{Ksp��CuS��}$=1.2��1012����1��105��
[��֪��Ksp��ZnS��=1.6��10-24��Ksp��CuS��=1.3��10-36��һ����Ϊ��Ӧ��ƽ�ⳣ��K��105���÷�Ӧ���л�����ȫ��]
��5������п�������ӷ���ʽΪ3Zn2++6HCO3-=2Zn��OH��2•ZnCO3��+5CO2��+H2O��
��6�����������ж����Ҫ���ˣ�ʵ���ҽ��й��˲�����Ҫ�õ��IJ����������ձ�����������©���������� III��������Һ��ѭ��ʹ�ã���Ʒ��ϴ�Ӹ�����ã������Ʒϴ�Ӹɾ��IJ�����������ȡ���һ��ϴ��Һ�������Թ��У����������ữ���Ȼ�����Һ���ް�ɫ�������ɣ�
���� �Ʊ�2Zn��OH��2•ZnCO3����Ϊ��п��ɰ����Ҫ�ɷ�ΪZnO��������Cu2+��Mn2+�����ӣ��м�������李���ˮ��˫��ˮ��˫��ˮ�������������ɶ������̣����˺�����Һ�м���泥��ٹ��ˣ���ȥͭ���ӣ�������ȥ����İ���������̼����淋õ�2Zn��OH��2•ZnCO3�Ͷ�����̼���壬���˵�2Zn��OH��2•ZnCO3����ҺΪ�������Һ��
��1����NH4��2SO4��NH3•H2O�Ļ����Һ�д��ڵ���غ㣺c ��NH4+��+c��H+��=2c��SO42-��+c��OH-�����ٽ��c��NH4+��=2c��SO42-���ж���Һ����ԣ�
��2������Ӱ�췴Ӧ���ʵ������жϡ���ȡ��ʱΪ�����п�Ľ����ʣ��ɲ�ȡ�Ĵ�ʩ��
��3������ȡ��ʱ�����NH3•H2O��������Һ�ʼ��ԣ�˫��ˮ��������������MnO2�����ݵ���غ��Ԫ���غ���д���ӷ���ʽ��
��4�����ݷ�ӦZnS+Cu2+=CuS+Zn2+����֪K=$\frac{Ksp��ZnS��}{Ksp��CuS��}$=1.2��1012�����������Ϣ���ж�ZnS���ӣ��Ƿ���У�
��5������п���Ĺ���Ϊ��Һ�е�п������̼�������Һ��Ӧ����2Zn��OH��2•ZnCO3���ݴ���д���ӷ���ʽ��
��6�����˲�����Ҫ�õ��IJ����������ձ�����������©���ȣ�����������������ӣ����ü�����������ӵķ��������Ƿ�ϴ�Ӹɾ���
��� �⣺��1��NH4��2SO4��NH3•H2O�Ļ����Һ�д��ڵ���غ㣺c ��NH4+��+c��H+��=2c��SO42-��+c��OH-������c��NH4+��=2c��SO42-��ʱ��c��H+��=c��OH-��������Һ�����ԣ�
�ʴ�Ϊ���У�
��2������Ӱ�췴Ӧ���ʵ������жϡ���ȡ��ʱΪ�����п�Ľ����ʣ��ɲ�ȡ�Ĵ�ʩΪ���衢�ʵ����ȣ�Ҳ���ӳ���ȡʱ�䡢��ν�ȡ��
�ʴ�Ϊ�����衢�ʵ����ȡ��ӳ���ȡʱ�䡢��ν�ȡ�ȣ�
��3������ȡ��ʱ�����NH3•H2O��������Һ�ʼ��ԣ�˫��ˮ��������������MnO2����Ӧ�����ӷ���ʽΪMn2++H2O2+2NH3•H2O=MnO2��+2NH4++2H2O��
�ʴ�Ϊ��Mn2++H2O2+2NH3•H2O=MnO2��+2NH4++2H2O��
��4�����ݷ�ӦZnS+Cu2+=CuS+Zn2+����֪K=$\frac{Ksp��ZnS��}{Ksp��CuS��}$=1.2��1012����1��105��K��105��ѧ��Ӧ��ȫ������ѡ��ZnS���г����ǿ��еģ�
�ʴ�Ϊ�����У�ZnS+Cu2+?CuS+Zn2+��K=$\frac{Ksp��ZnS��}{Ksp��CuS��}$=1.2��1012����1��105��
��5������п���Ĺ���Ϊ��Һ�е�п������̼�������Һ��Ӧ����2Zn��OH��2•ZnCO3�����ӷ���ʽΪ3Zn2++6HCO3-=2Zn��OH��2•ZnCO3��+5CO2��+H2O��
�ʴ�Ϊ��3Zn2++6HCO3-=2Zn��OH��2•ZnCO3��+5CO2��+H2O��
��6�����˲�����Ҫ�õ��IJ����������ձ�����������©���ȣ�����������������ӣ����ü�����������ӵķ��������Ƿ�ϴ�Ӹɾ���������ȡ���һ��ϴ��Һ�������Թ��У����������ữ���Ȼ�����Һ���ް�ɫ�������ɣ�
�ʴ�Ϊ���ձ�����������©����ȡ���һ��ϴ��Һ�������Թ��У����������ữ���Ȼ�����Һ���ް�ɫ�������ɣ�
���� ���⿼���������Ʊ���������ơ����ʷ������ᴿ�������ۺ�Ӧ�ã�Ϊ��Ƶ����ͳ������ͣ���Ŀ�ѶȽϴ���ȷ�Ʊ�����Ϊ���ؼ���ע�����ճ������ʷ������ᴿ�IJ�������������֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ�����ѧ���ķ�����������������ѧʵ��������

 �����ߴ���ϵ�д�
�����ߴ���ϵ�д���l���Ʊ�𩷯��ʵ��������ͼ��ʾ��
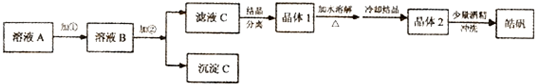
��֪�������������↑ʼ���ɳ�����������ȫ��pH��Χ�ֱ�Ϊ��
| ��ʼ���� | ������ȫ | |
| Fe��OH��3 | 2.7 | 3.7 |
| Fe��OH��2 | 7.6 | 9.6 |
| Zn��OH��2 | 5.7 | 8.0 |
�ɹ�ѡ��ʹ�õ��У���ˮ��20%��H2O2����ˮ��NaOH��Һ��Zn��OH��2��ZnO
�ټ�����Լ��٣�Ӧѡ��20%��H2O2��������Ӧ�����ӷ���ʽΪ2H++H2O2+2Fe2+=2Fe3++2H2O
�ڼ�����Լ��ڣ���ѡ��ZnO��Zn��OH��2���������ǵ�����Һ��pH��3.7-5.7��ʹFe3+��ȫת��ΪFe��OH��3������ͬʱ���������µ�����
�۴Ӿ���l������2�����ᴿ���̵��������ؽᾧ��
����ͬѧ�����Ӧ������1����ϡ�������ܽ⣬�������ӷ���ʽ����Zn2++2H2O?Zn��OH��2+2H+��ϡ���������ZnSO4��ˮ�⣬��ֹ����Zn��OH��2���ʣ�
���ڵõ�𩷯ʱ�������м��������ƾ�ϴ�Ӷ�����ˮ��ԭ����Ϊ�˳�ϴ����������������Һ����ֹ�����ܽ⣬Ӱ����ʣ�
��2������𩷯���Ƿ���FeԪ�صIJ������Լ���ѡ��ȡ����𩷯����ˮ���Ƴ���Һ����������Һ�еμ�����KSCN��Һ��������������˵������Fe3+������Һ��Ϊ��ɫ��˵����Fe3+��
| A�� | �����һ���Ǵ����� | |
| B�� | �������ǹ������ƵĻ���� | |
| C�� | Na+��AlO2-��SiO32-һ����������ҺX�� | |
| D�� | CO32-��SO42-һ������������ҺX�� |
| A�� | 1��1-������ϩ | B�� | ��ϩ | C�� | 1-��ϩ | D�� | 2-��ϩ |
| A�� | �ô�����Һ�ܽⱽ�ӣ�CO32-+C6H5OH��C6H5O-+HCO3- | |
| B�� | ��ǿ����Һ���չ�ҵ��ȡ����β��NO+NO2+2OH-=2NO3-+H2O | |
| C�� | �ö������̺�Ũ���ᷴӦ��������MnO2+4HCl��Ũ�� $\frac{\underline{\;\;��\;\;}}{\;}$Mn2++2Cl-+Cl2��+2H2O | |
| D�� | ��AlCl3��Һ�еμӹ����İ�ˮ��Al3++4NH3•H2O=AlO2-+2H2O |
| A�� | �ڸ÷�Ӧ�����£�Mg�Ļ�ԭ��ǿ��C�Ļ�ԭ�� | |
| B�� | Mg��MgO��þԪ�����İ뾶��r��Mg2+����r��Mg�� | |
| C�� | Ԫ��C�ĵ���ֻ���ڽ��ʯ��ʯī����ͬ�������� | |
| D�� | �÷�Ӧ�л�ѧ��ȫ��ת��Ϊ���� |
 ��ش��������⣺
��ش��������⣺