��Ŀ����
4�� ij��ѧ��ȤС���ʵ�����Ҵ�������ʵ��װ�ý����˸Ľ�����ͼ���Թ�A��Ϊ������ع��壬�Թ�B��Ϊ��ˮ�Ҵ���Ӳ���Թ�C��Ϊ�Ƴ�����״��ϸ��˿�������D��Ϊ��ˮ����ͭ��ĩ����ͼ�м����������г�װ�õȶ�����ȥ��
ij��ѧ��ȤС���ʵ�����Ҵ�������ʵ��װ�ý����˸Ľ�����ͼ���Թ�A��Ϊ������ع��壬�Թ�B��Ϊ��ˮ�Ҵ���Ӳ���Թ�C��Ϊ�Ƴ�����״��ϸ��˿�������D��Ϊ��ˮ����ͭ��ĩ����ͼ�м����������г�װ�õȶ�����ȥ�����ʴ��������⣺
��1��������װ���У�ʵ��ʱ��ˣ���ȵ�������λ��ABC�����������λ��Ӧ����ĸ����
��2��ΪʹB���Ҵ�ƽ����������������ʹ�õķ����Dz���ˮԡ���ȣ�C����Ӧ�Ļ�ѧ����ʽΪ2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��
��3���÷�ӦΪ���ȷ�Ӧ����Ӧһ��ʱ�����C���ľƾ��ƣ�C���������Ǻ��ڱ�죬D����˿ɹ۲쵽��ɫ��ĩ��ˮ����ͭ����ɫ����4����֪��ȩ�е�Ϊ20.8�棬�ӷ�������E��Ӧʢ�ű�ˮ�����Ա���F���ռ���Һ�����ȩ��
��5������װ������һ�������ڰ�ȫ��������ָ��������Ľ�������
����֮����E�е��Թܴ��е����Թ�����
�Ľ���������Ϊ˫���Թ�����������С���ܣ�
���� ��1������ʵ�����̺ͷ�Ӧԭ������A�Ǽ�����������B��Ҫ�õ��Ҵ�������Ҫ���ȣ��Ҵ��Ĵ�������Ҫ���ȣ�
��2��ˮԡ���ȿ���ʹB���Ҵ�ƽ����������������C���Ҵ�������Ϊ��ȩ��ˮ��
��3���Ҵ�������Ϊ��ȩ��ˮ������ͭ���ڱ�죬��ɫ��ĩ��ˮ����ͭ��ˮ����ɫ��
��4����ȩ�е�Ϊ20.8�棬���Խ����¶ȿ�������ȩ��Һ����ڣ�
��5��E�е��Թ��������ȩ��Ӧ�ü�һ��С�̵��ܣ���֤��ѹ��ͨ��
��� �⣺��1��װ���з�Ӧ��������Ҫ�Ʊ��������Ҵ������ڼ���ʱ��Ӧ������ȩ��������Ҫ���ȵ�װ���У�A�Ǽ�����������B��Ҫ�õ��Ҵ�������Ҫ���ȣ�C���Ҵ��Ĵ�������Ҫ���ȣ���ѡABC��
��2��ˮԡ���ȿ���ʹB���Ҵ�ƽ����������������C���Ҵ�������Ϊ��ȩ��ˮ����2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O���ʴ�Ϊ������ˮԡ���ȣ�2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��
��3���Ҵ�������Ϊ��ȩ��ˮ������ͭ���DZ�����Ϊ����ͭ��Ȼ������ͭ����ԭΪ����ͭ������ͭ�Ǵ�����������ͭ����ɫ�仯�����ڱ�죬C���������Ǻ��ڱ��
���Ҵ�������Ϊ��ȩ��ˮ��D����˿ɹ۲쵽��ɫ��ĩ��ˮ����ͭ��ˮ����ɫ���ʴ�Ϊ�����ڱ�죻��ɫ��ĩ��ˮ����ͭ����ɫ��
��4����ȩ�е�Ϊ20.8�棬���Խ����¶ȿ�������ȩ��Һ����ڣ�ʢ�ŵ��DZ�ˮ����
��5��E�е��Թܴ��е����Թ�������ѹ����ͨ���������ȩ��Ӧ�ü�һ��С�̵��ܣ���֤��ѹ��ͨ��
�ʴ�Ϊ��E�е��Թܴ��е����Թ�������Ϊ˫���Թ�����������С���ܣ�
���� ���⿼�����������ʵ�ʵ����֤��ʵ�鷽����Ʒ�����ʵ�������жϣ���Ӧԭ����Ӧ�ã������������ʺ�ʵ����������ǽ���ؼ�����Ŀ�Ѷ��еȣ�

 ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�| A�� | �ƵĽ����������ͭǰ�棬���Խ����ƿ����û�������ͭ��Һ�е�ͭ | |
| B�� | ��������ʢ�ź�����Ũ���� | |
| C�� | ��������ˮ�γɵ�Al��OH��3����������ˮ�������������ˮ�ľ��� | |
| D�� | ��������Ʒ���渲��������Ĥ�����ڲ������𱣻����� |
 ����ƽ�ⳣ������Ka��ʾ���Ĵ�С�����жϵ���ʵ����ǿ����25��ʱ���й����ʵĵ���ƽ�ⳣ�����±���ʾ��
����ƽ�ⳣ������Ka��ʾ���Ĵ�С�����жϵ���ʵ����ǿ����25��ʱ���й����ʵĵ���ƽ�ⳣ�����±���ʾ��| ��ѧʽ | HF | H2CO3 | HClO |
| ����ƽ�ⳣ����Ka�� | ��7.2��10-4 | K1=4.4��10-7 K2=4.7��10-11 | ��3.0��10-8 |
��H+��aq��+OH-��aq���TH2O��l����H=-57.3 kJ/mol��
�����ĵ��뷽��ʽ����ЧӦ�ɱ�ʾΪHF��aq��?H+��aq��+F-��aq����H=-10.4kJ•mol-1��
��2����Ũ��Ϊ0.1 mol/L HF��Һ��ˮϡ��һ���������¶Ȳ��䣩�����и����������CD��
A��c��H+��������������������������������B��c��H+��•c��OH-��
C��$\frac{c��{H}^{+}��}{c��{H}^{-}��}$������������������������������D��$\frac{c��O{H}^{-}��}{c��{H}^{+}��}$
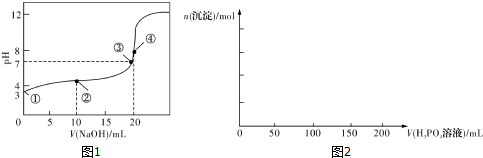
��3����20 mL 0.1 mol/L������м���V mL 0.1 mol/L NaOH��Һ����û����Һ��pH�仯������ͼ��ʾ������˵����ȷ����BC��
A��pH=3��HF��Һ��pH=11��NaF��Һ�У���ˮ�������c��H+�����
B���ٵ�ʱpH=6����ʱ��Һ�У�c��F-��-c��Na+��=9.9��10-7mol/L
C���ڵ�ʱ����Һ�е�c��F-��=c��Na+��
D���۵�ʱV=20 mL����ʱ��Һ��c��F-����c��Na+��=0.1 mol/L
��4�����ʵ���Ũ�Ⱦ�Ϊ0.1 mol/L������������Һ����Na2CO3��Һ����NaHCO3��Һ����NaF��Һ��NaClO��Һ�����������ж�pH�ɴ�С��˳���Ǣ٣��ܣ��ڣ��ۣ�
��5��Na2CO3��Һ�Լ�������ΪCO${\;}_{3}^{2-}$ˮ���Ե�ʣ�����Ƽ�ʵ����ʵ֤��֮��̼������Һ�е����̪��Һ��죬�ټ���BaCl2��Һ����������Һ�ɫ��ȥ���dz��
��6������������һֱ��Ϊ���ĺ�������ڣ�1971��������ѧ���÷���ͨ��ϸ��ĩʱ���HFO����ṹʽΪH-O-F��HFO��ˮ��Ӧ�õ�HF�ͻ�����A��ÿ����1 mol HFת��1mol���ӣ�
| A�� | Cu+4HNO3�TCu��NO3��2+2NO2��+2H2O | B�� | 2NaOH+CuSO4�TNa2SO4+Cu��OH��2�� | ||
| C�� | 2CO+O2�T2CO2 | D�� | 2Al+2NaOH+2H2O�T2NaAlO2+3H2�� |
��2������Na2SO3��Һ����SO2�Ĺ����У�pH��n��SO32-����n��HSO3-���仯��ϵ���±���
| n��SO32-����n��HSO3-�� | 1��9 | 1��1 | 1��91 |
| pH | 8.2 | 7.2 | 6.2 |
��3����0.1mol•L-1��NaHSO3��ͨ�˰�������Һ������ʱ����Һ�е�c��H+����c��OH-����c��SO32-����c��Na+����c��NH4+������������Ũ�ȴ�С��ϵ��c��Na+����c��SO32-����c��NH4+����c��H+��=c��OH-����
��4����֪Ca3��PO4��2��CaHPO4��������ˮ����Ca��H2PO4��2���ܣ��ں�0.1molCa��OH��2�ij���ʯ��ˮ����μ���1mol•L-1��H3PO4������ͼ2���������ɳ��������ʵ�����H3PO4���������0��ʼ��200mL��ͼ��
| A�� | һ�������£���1 mol N2��3 mol H2��ϣ���ַ�Ӧ��ת�Ƶĵ�����Ϊ6 NA | |
| B�� | 1.5 mol NO2������ˮ��Ӧ��ת�Ƶĵ�����Ϊ1.5 NA | |
| C�� | 6.4 g��S2��S4��S8��ɵĻ���ﺬ��ԭ����Ϊ0.2 NA | |
| D�� | ���³�ѹ�£�11.2 L Cl2����ԭ����ΪNA |
 ��͵�Ԫ���ڻ�ѧ���к���Ҫ�ĵ�λ���ش��������⣺
��͵�Ԫ���ڻ�ѧ���к���Ҫ�ĵ�λ���ش��������⣺ ��Ԥ����2017�귢��ġ��϶���š�̽�������õij���5�����ػ��ȼ��Ϊƫ������[��CH3��2NNH2]����CH3��2NNH2��Nԭ�ӵ��ӻ���ʽΪsp3��
��Ԥ����2017�귢��ġ��϶���š�̽�������õij���5�����ػ��ȼ��Ϊƫ������[��CH3��2NNH2]����CH3��2NNH2��Nԭ�ӵ��ӻ���ʽΪsp3�� ��
��