��Ŀ����
ij����С����Ƶ�ʵ����������������װ������ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ��
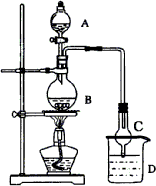
��֪��
����ˮ�Ȼ��ƿ����Ҵ��γ�������ˮ��CaCl2��6C2H5OH��
���й��л���ķе����£�
��ش�
(1)Ũ�����������________��
(2)���θ����C��������________����Ӧ������D�е�����________________��
(3)��D�з�����������������г�����һ���������ᡢ�Ҵ���ˮ��Ӧ�ȼ���������ˮ�Ȼ��ƣ������________��Ȼ����������ռ�77�����ҵ���֣��Եõ��ϴ���������������
������
|
����(1)��������ˮ��(3��)�� ����(2)������������(2��)��Һ���Ϊ���㣬�ϲ������Ĺ���ζ����״Һ�塡(2��) ����(3)�Ҵ���ˮ��(2��) |

 I������ʵ����ơ�������ʵ�������������
I������ʵ����ơ�������ʵ�������������A������ά�غ������Ϲ��Ⱥ��Һ�壬ȡ���������������Ƶ�Cu��OH��2����Һ���ȣ��۲��Ƿ���ש��ɫ�������ɣ���֤����ά��ˮ������������
B��Һ̬�������м���ϡNaOH��Һ�����ӣ�Ȼ���������ϡHNO3���ټ���AgNO3��Һ����Br-������
C������������Ƿ���м�ȩ��������Ʒ�м�������NaOH��Һ���к�HCOOH������������Ӧʵ��
D�����뱽�ͱ��ӵĻ��Һ����������Ũ��ˮ�����ˣ����ɷ���
E����ͭ˿�ھƾ��������ϼ��Ⱥ�����������ˮ�Ҵ��У�ͭ˿�ָ���ԭ���ĺ�ɫ
F����ȡ�ܽ���ˮ�е������⣺����ƾ��������÷ֲ��ȡ���л����ٷ���
G�����Թ��м���2mL10%��CuSO4��Һ������2%��NaOH��Һ4��6�Σ��������ȩ��Һ0.5mL�����������ڣ�֤����ȩ������
����֪������ˮ�Ȼ��ƿ����Ҵ��γ�������ˮ��CaCl2?6C2H5OH��
���й��л���ķе㣺
| �Լ� | ���� | �Ҵ� | ���� | �������� |
| �е㣨�棩 | 34.7 | 78.5 | 118 | 77.1 |
��1��A��Ũ�����������
��2������ͬλ��18Oʾ�ٷ�ȷ����Ӧ����ˮ��������ԭ�ӵ��ṩ�ߣ�д���ܱ�ʾ18Oλ�õĻ�ѧ��Ӧ����ʽ
��3����ʵ����ʹ�����ιܳ������������⣬��һ��Ҫ������
��4����Ӧ������D�е�������
��5����B�з���������������л�����һ�������Ҵ������Ѻ�ˮ��Ӧ�ȼ�����ˮ�Ȼ��ƣ����˷����
A ���������� B ��ʯ�� C ��ˮ������ D ��ʯ�ң�
��֪������ˮ�Ȼ��ƿ����Ҵ��γ�������ˮ��CaCl2��6C2H5OH.
���й��л���ķе���±���
|
�Լ� |
���� |
�Ҵ� |
���� |
�������� |
|
�е�(��) |
34.5 |
78.3 |
118 |
77.1 |
ij����С����Ƶ�ʵ������ȡ�϶�������������װ����ͼ��ʾ��A�з����Ҵ�����ˮ�����Ũ���ᣬB�з��б���̼������Һ���Իش�

(1)A��Ũ�����������________������ͬλ��18Oʾ�ٷ�ȷ����Ӧ����ˮ��������ԭ�ӵ��ṩ�ߣ�д���ܱ�ʾ18Oλ�õĻ�ѧ����ʽ��______________________.
(2)B�����ιܵ�����������________��________.����Ӧǰ��B����Һ�μӼ��η�̪���ʺ�ɫ�������������ԭ����__________________________(�����ӷ���ʽ��ʾ)����Ӧ������B�е�������______________________________��


 ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ����ش�
ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ����ش�