��Ŀ����
9��������Һ������Ũ�ȹ�ϵ�ı�ʾ��ȷ���ǣ�������| A�� | NaHCO3��Һ�У�c��H+��+c��Na+���Tc��OH-��+c��CO32-��+c��HCO3-�� | |
| B�� | pH=3��CH3COOH��Һ��pH=11��NaOH��Һ�������Ϻ����Һ�У�c��OH-����c��H+�� | |
| C�� | 0.1mol/L��NH4Cl��Һ�У�c��Cl-����c��H+����c��NH4+����c��OH-�� | |
| D�� | ���ʵ���Ũ����ȵ�CH3COOH��CH3COONa��Һ�������Ϻ����Һ�У�2c��Na+���Tc��CH3COOH��+c��CH3COO-�� |
���� A������̼��������Һ�еĵ���غ��жϣ�
B������Ϊ���ᣬ���Һ�д����������Һ�����ԣ���c��OH-����c��H+����
C��笠����ӵ�ˮ��̶Ƚ�С����c��NH4+����c��H+����
D�����ݻ��Һ�е������غ��жϣ�
��� �⣺A��NaHCO3��Һ�У����ݵ���غ��֪����c��H+��+c��Na+���Tc��OH-��+2c��CO32-��+c��HCO3-������A����
B��pH=3��CH3COOH��Һ��pH=11��NaOH��Һ�������ϣ����ڴ���Ϊ���ᣬ������������Һ��ʾ���ԣ�c��OH-����c��H+������B����
C��0.1mol/L��NH4Cl��Һ�У�笠�����ˮ�⣬��Һ�����ԣ�����ˮ��̶Ƚ�С����c��NH4+����c��H+������ȷ������Ũ�ȴ�СΪ��c��Cl-����c��NH4+����c��H+����c��OH-������C����
D�����ʵ���Ũ����ȵ�CH3COOH��CH3COONa��Һ�������ϣ����������غ�ɵã�2c��Na+���Tc��CH3COOH��+c��CH3COO-������D��ȷ��
��ѡD��
���� ���⿼��������Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ���ȷ�ε�ˮ��ԭ������Ӱ��Ϊ���ؼ���ע�����յ���غ㡢�����غ�ĺ��弰Ӧ�÷���������������ѧ���ķ������������Ӧ��������

 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�| A�� | ��NaOH��Һ����ε��뱥���Ȼ�����Һ���Ʊ�Fe��OH��3���� | |
| B�� | ��Fe��OH��3��������ε���ϡ���ᣬ�������ȳ��ֺ��ɫ���������ܽ�תΪ��ɫ��Һ | |
| C�� | ���������ЧӦ�����ֽ������Һ��Ψһ�ֶ� | |
| D�� | ��������뽺�������� |

| A�� | ����ͼ���ж�����ӦΪ���ȷ�Ӧ | |
| B�� | ��ͼ���У����߿ɱ�ʾʹ���˴��� | |
| C�� | ������Ӧ�ġ�H��0��ͼ���ɱ�ʾ�����¶�ʹƽ�����淴Ӧ�����ƶ� | |
| D�� | ��ͼ��������ƽ����Է����������¶ȵı仯���������֪����Ӧ�ġ�H��0 |
| A�� | SO42- | B�� | Fe2+ | C�� | S2- | D�� | OH- |
| A�� | N2 | B�� | MgCl2 | C�� | Na2O | D�� | Na2O2 |
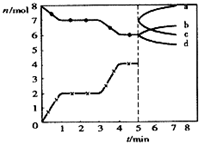 �����Ϊ2L���ܱ������н������з�Ӧ��C��s��+CO2��g���T2CO��g������H=+Q kJ•mol-1����ͼΪCO2��CO�����ʵ�����ʱ��t�ı仯��ϵͼ������˵������ȷ���ǣ�������
�����Ϊ2L���ܱ������н������з�Ӧ��C��s��+CO2��g���T2CO��g������H=+Q kJ•mol-1����ͼΪCO2��CO�����ʵ�����ʱ��t�ı仯��ϵͼ������˵������ȷ���ǣ�������| A�� | ��0-1min��CO�����ʵ���������2mol | |
| B�� | ���̽�̿�������������仯ʱ��˵����Ӧ�Ѵ�ƽ��״̬ | |
| C�� | 5minʱ�ٳ���һ������CO��n��CO����n��CO2���ı仯�ɷֱ���c��b���߱�ʾ | |
| D�� | 3minʱ�¶���T1���ߵ�T2������ƽ��ʱK��T2��С��K��T1�� |
| A�� | ����������������θ���кͼ� | |
| B�� | ˮ���������������ϼ��ͷ���� | |
| C�� | ����������Һ�����ڻ���������ɱ�� | |
| D�� | ��������ɹ㷺����ʳƷ��Ư�� |
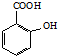 ����Ӧ�ܵõ���ѧʽΪC7H5O3Na����A
����Ӧ�ܵõ���ѧʽΪC7H5O3Na����A