��Ŀ����
ijѧ����Ũ�������ʵ�ʵ�飺
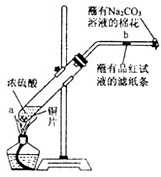
��һ֧�Թ��з���һ���С��ͭƬ���ټ���2mLŨ���ᣬȻ����Թ̶ܹ�������̨�ϣ���һС��պ��Ʒ����Һ����ֽ������е�����Ƥ���IJ������У������Թܿڣ��ڲ����ܿڴ�����һ��պ��NaOH��Һ�����������Թܣ��۲�����
�ش��������⣺
��1��д���Թ��з�����Ӧ�Ļ�ѧ����ʽ______��
��2���Թ��е�Һ�巴Ӧһ��ʱ���b����ֽ���ı仯Ϊ______�����Թ��з�Ӧֹͣ�������ܷ���պ��Ʒ����Һ����ֽ�����ȣ���ֽ���ı仯Ϊ______��
��3��պ��NaOH��Һ������������______��
��4��������������γɹ��̿������з�Ӧ�е�______����ʾ��
A��2SO2+O2
2SO3
B��SO3+H2O�TH2SO4
C��SO2+H2O2�TH2SO4��
��һ֧�Թ��з���һ���С��ͭƬ���ټ���2mLŨ���ᣬȻ����Թ̶ܹ�������̨�ϣ���һС��պ��Ʒ����Һ����ֽ������е�����Ƥ���IJ������У������Թܿڣ��ڲ����ܿڴ�����һ��պ��NaOH��Һ�����������Թܣ��۲�����
�ش��������⣺
��1��д���Թ��з�����Ӧ�Ļ�ѧ����ʽ______��
��2���Թ��е�Һ�巴Ӧһ��ʱ���b����ֽ���ı仯Ϊ______�����Թ��з�Ӧֹͣ�������ܷ���պ��Ʒ����Һ����ֽ�����ȣ���ֽ���ı仯Ϊ______��
��3��պ��NaOH��Һ������������______��
��4��������������γɹ��̿������з�Ӧ�е�______����ʾ��
A��2SO2+O2
| ���� |
B��SO3+H2O�TH2SO4
C��SO2+H2O2�TH2SO4��

��1��ͭ��Ũ�����ڼ����������ܷ�����Ӧ��Ũ�������ǿ�����ԣ���ͭ��ԭΪ�����������Բ��������ɵ�����ͭ�����������ˮ����д��ѧ����ʽΪCu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O��
�ʴ�Ϊ��Cu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O
��2�����������ܺ���ɫ����������ɫ���ʣ�����������ʹƷ����Һ��ɫ�����Զ����������Ư���ԣ������ɵ���ɫ���ʲ��ȶ�������ʱ��ָ�ԭ������ɫ��
�ʴ�Ϊ��Ʒ����Һ��ɫ����Һ�ָ���ɫ��
��3�����������ж������Բ���ֱ���ſգ���������������������ܺͼӦ�����κ�ˮ�������Թܢ�ܿ���һ�Ž���
NaOH��Һ����������������δ��Ӧ��SO2���壬������Ӧ�����ӷ���ʽΪSO2+2OH-=SO32-+H2O��
�ʴ�Ϊ������δ��Ӧ��SO2���壬��ֹ��Ⱦ������
��4��������������γɹ��̣����ø����ȴ������°Ѷ�����������Ϊ������������ˮ�γ��������AB��Ӧ���ϣ�
�ʴ�Ϊ��AB��
| ||
�ʴ�Ϊ��Cu+2H2SO4��Ũ��
| ||
��2�����������ܺ���ɫ����������ɫ���ʣ�����������ʹƷ����Һ��ɫ�����Զ����������Ư���ԣ������ɵ���ɫ���ʲ��ȶ�������ʱ��ָ�ԭ������ɫ��
�ʴ�Ϊ��Ʒ����Һ��ɫ����Һ�ָ���ɫ��
��3�����������ж������Բ���ֱ���ſգ���������������������ܺͼӦ�����κ�ˮ�������Թܢ�ܿ���һ�Ž���
NaOH��Һ����������������δ��Ӧ��SO2���壬������Ӧ�����ӷ���ʽΪSO2+2OH-=SO32-+H2O��
�ʴ�Ϊ������δ��Ӧ��SO2���壬��ֹ��Ⱦ������
��4��������������γɹ��̣����ø����ȴ������°Ѷ�����������Ϊ������������ˮ�γ��������AB��Ӧ���ϣ�
�ʴ�Ϊ��AB��

��ϰ��ϵ�д�
�����Ŀ
 ijѧ����Ũ�������ʵ�ʵ�飺
ijѧ����Ũ�������ʵ�ʵ�飺

 ijѧ����Ũ�������ʵ�ʵ�飺
ijѧ����Ũ�������ʵ�ʵ�飺


