��Ŀ����
13��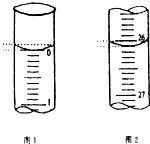 ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ�������ָʾ��������д���пհף�
ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ�������ָʾ��������д���пհף���1���ñ���������Һ�ζ����������������Һʱ�����ְ�����ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�ע����ƿ����Һ��ɫ�ı仯��ֱ�������һ���������Һ�ɻ�ɫ��Ϊ��ɫ�������������ɫ����Ϊֹ��
��2�����в����п���ʹ��������������Һ��Ũ����ֵƫ�͵���D
��A����ʽ�ζ���δ�ñ�������Һ��ϴ��ֱ��ע���������Һ
��B���ζ�ǰʢ������������Һ����ƿ������ˮϴ����û�и���
��C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
��D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
��3�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ������ʼ����Ϊ0.00mL���յ����Ϊ26.10mL������������Һ�����Ϊ26.10 mL��
��4��ijѧ����������ʵ��ֱ��¼�й����������
| �ζ����� | ��������������Һ�����/mL | 0.1000mol•L-1��������/mL | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
| ��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
| �ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
| ������ | 25.00 | 0.22 | 26.31 | 26.09 |
���� ��1�����ְ�����ʽ�ζ��ܵĻ���������ҡ����ƿ���۾��۾�Ҫע����ƿ����Һ����ɫ�仯���ζ��յ�ʱ��Һ��ɫ�ɺ�ɫͻ��Ϊ��ɫ��
��2������c�����⣩=$\frac{c������V������}{V�����⣩}$��������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��3����ʽ�ζ��ܵ�С�̶����Ϸ���ȷ��Ϊ0.01mL��
��4���ȸ������ݵ���Ч�ԣ���ȥ��2�����ݣ�Ȼ�����1��3��ƽ������V�����ᣩ�����Ÿ��������NaOH��Ӧ���c��NaOH����
��� �⣺��1������к͵ζ�ʱ���۾�Ҫע����ƿ����Һ����ɫ�仯���ζ��յ�ʱ��Һ��ɫ�ɺ�ɫͻ��Ϊ��ɫ���Ұ��������ɫ���䣬
�ʴ�Ϊ����ƿ����Һ��ɫ�ı仯�����������ɫ���䣻
��2��A����ʽ�ζ���δ�ñ�������Һ��ϴ��ֱ��ע���������Һ����Һ��ϡ�ͣ����V������ƫ����c�����⣩=$\frac{c������V������}{V�����⣩}$��֪���ⶨc��NaOH��ƫ��A����
B���ζ�ǰʢ������������Һ����ƿ������ˮϴ����û�и������Һ�����ʵ������䣬��V��������Ӱ�죬����c�����⣩=$\frac{c������V������}{V�����⣩}$��֪���ⶨc��NaOH����Ӱ�죬��B����
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ�����V������ƫ����c�����⣩=$\frac{c������V������}{V�����⣩}$��֪���ⶨc��NaOH��ƫ��C����
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ��������V������ƫС������c�����⣩=$\frac{c������V������}{V�����⣩}$��֪���ⶨc��NaOH��ƫС����D��ȷ��
�ʴ�Ϊ��D��
��3����ͼ��֪����ʼ����Ϊ0.00mL���յ����Ϊ26.10mL������������Һ�����Ϊ26.10mL-0.00mL=26.10 mL��
�ʴ�Ϊ��0.00��26.10��26.10��
��4���������ݵ���Ч�ԣ���ȥ��2�����ݣ���1��3�����ݿ�֪ƽ������V�����ᣩ=$\frac{26.11+26.09}{2}$mL=26.10mL�����ݷ�Ӧ����ʽ��HCl+NaOH=NaCl+H2O��n��HCl��=n��NaOH��������0.0261L��0.1000mol•L-1=0.025L��c��NaOH�������c��NaOH��=$\frac{0.0261L��0.1000mol/L}{0.025L}$=0.1044mol/L��
�ʴ�Ϊ��0.1044mol/L��
���� ���⿼�����ʺ����ⶨʵ�飬Ϊ��Ƶ���㣬����������ʹ�á��к͵ζ���Ӧ�õ�Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬ע�⣨4�������ݴ�������Ŀ�ѶȲ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�������0.50mol•L-1NaOH��Һ
��1����ʵ���д�ԼҪʹ��470mL NaOH��Һ��������Ҫ����NaOH����10.0 g��
��2����ͼ��ѡ�����NaOH��������Ҫ�������ǣ�����ĸ����a��b��e��
| ���� | ������ƽ | С�ձ� | ����ǯ | ������ | ҩ�� | ��Ͳ |
| ���� | 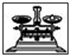 |  |  | 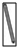 |  | |
| ��� | a | b | c | d | e | f |
��2��ȡ50mL NaOH��Һ��30mL������Һ����ʵ�飬ʵ���������±���������д�±��еĿհף�
| ʵ�� ���� | ��ʼ�¶�t1/�� | ��ֹ�¶� t2/�� | �¶Ȳ�ƽ��ֵ ��t2-t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 30.1 | |
| 2 | 27.0 | 27.4 | 27.2 | 33.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |
������ʵ����ֵ�����57.3kJ•mol-1��ƫ�����ƫ���ԭ������ǣ�����ĸ��A��C��D��
A��ʵ��װ�ñ��¡�����Ч����
B����ȡNaOH��Һ�����ʱ���Ӷ���
C���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
D�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶ȣ�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����ʳ�����������/mL | 0.02 | 0.03 | 0.00 |
| ����ʳ������ն���/mL | 25.01 | 25.04 | 25.02 |
| �������Ʊ�Һ�����������/mL | 0.01 | 0.03 | 0.04 |
| �������Ʊ�Һ������ն���/mL | 12.52 | 12.55 | 12.58 |
 ��֪��Ӧ��3I-��aq��+S2O82-��aq���TI3-��aq��+2SO42-��aq��+Q
��֪��Ӧ��3I-��aq��+S2O82-��aq���TI3-��aq��+2SO42-��aq��+Q��1��д����Ӧ��ƽ�ⳣ������ʽ��K=$\frac{{c}^{2}��S{{O}_{4}}^{2-}��c��{{I}_{3}}^{-}��}{{c}^{3}��{I}^{-}��c��{S}_{2}{{O}_{8}}^{2-}��}$��
��2����ͼ��ʾ��Ӧ�������й����ʵ���������Ӧ�����е�Q��0���������=������I������II���������У�ʹ�ô������ǣ�II�����ߣ�
��3����Ӧ�����ʿ�����I3-�����ĵ�����Һ��Ӧ����ɫ��ʱ��t��������tԽС����Ӧ����Խ���������20�����ʵ��ʱ����¼������
| ʵ���� | �� | �� | �� | �� | �� |
| c��I-��/mol•L-1 | 0.040 | 0.080 | 0.080 | 0.160 | 0.160 |
| c��S2O82-��/mol•L-1 | 0.040 | 0.040 | 0.080 | 0.080 | 0.040 |
| t/s | 88 | 44 | 22 | 11 | t1 |
| A�� | 1 mol�����к���1.204��1024����ԭ�ӣ��ڱ�״����ռ�����22.4 L | |
| B�� | 1 mol������1.5 mol����������ͬ����ԭ���� | |
| C�� | ��He��H2��O2����������¶Ⱥ��ܶȶ���ͬʱ�����ǵ�ѹǿ��С��p��O2����p��He����p��H2�� | |
| D�� | �����ʵ����ĸɱ��������ǣ�����ʽΪC6H12O6��������̼ԭ����֮��Ϊ1��6����ԭ����֮��Ϊ1��3 |
| A�� | ��������ķ�Һ����ˮ�ۣ���ˮ������ˮ�� | |
| B�� | ��ҽ�þƾ�������������ɵõ����ߴ��ȵľƾ���Һ | |
| C�� | ���ȸ�����ع�����ȡ���ռ�����������Ӧ����ֹͣ���� | |
| D�� | ����ʵ��ʱ����ˮӦ���Ͽڽ��¿ڳ� |
 ������̼�IJ�����������Դ�����һ����Ҫս�Է���
������̼�IJ�����������Դ�����һ����Ҫս�Է��� ��������ѧ���������ǹ��ۼ���
��������ѧ���������ǹ��ۼ���
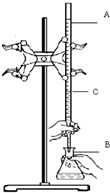 ��ʵ��������֪Ũ�ȵ�����ζ�ijδ֪Ũ�ȵ�NaOH��Һ��װ�úͲ�������ͼ��ʾ����ش�
��ʵ��������֪Ũ�ȵ�����ζ�ijδ֪Ũ�ȵ�NaOH��Һ��װ�úͲ�������ͼ��ʾ����ش�