��Ŀ����
ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
�ٽ���ʽ�ζ���������ˮϴ������ע�������Һ�����ڵζ��ܵļ��첿�ֳ�����Һ����ʹҺ�洦�ڡ�0���̶����µ�λ�ã����¶���������ƿ������ˮϴ���Ӽ�ʽ�ζ����з���20.00mL������Һ����ƿ�У�
�ڽ���ʽ�ζ���������ˮϴ�������ñ���Һ��ϴ2-3�κ�������ע��0.1000mol-L-1�����ᣬ���ڵζ��ܵļ��첿�ֳ�����Һ����ʹҺ�洦�ڡ�0���̶����µ�λ�ã����¶�����
������ƿ�е���ָʾ�������еζ����ζ����յ㣬��¼���ݣ�
���ظ����Ϲ���2�Σ��Իش��������⣺
��1��Ӧ��NaOH��Һע��ͼ1-�е�______��ѡ��ס����ҡ����У�
��2����С���ڲ�����еĴ�����______���ɴ���ɵIJⶨ���______��ƫ�ߡ�ƫ�ͻ���Ӱ�죩��
��3����ͼ2��ij�εζ�ʱ�ĵζ����е�Һ�棬ͼ2��ʾ50mL�ζ�����Һ���λ�ã���A��C�̶ȼ����1mL��A���Ŀ̶�Ϊ25���ζ�����Һ�����ӦΪ______mL��
��4���õζ������Т�Ӧѡ�õ�ָʾ����______���ζ�ʱ�����ֿ��Ƶζ��ܻ����������ճ���ƿ���ߵα����۾�ע�ӣ�______�����ȷ���յ㣿______��
��5�������������ݣ�
���������ռ���Һ��Ũ��Ϊ______��
��6������1mol/L��0.1mol/L��HCl��Һ��Ӧ��______��HCl��Һ��ԭ����______��
�ٽ���ʽ�ζ���������ˮϴ������ע�������Һ�����ڵζ��ܵļ��첿�ֳ�����Һ����ʹҺ�洦�ڡ�0���̶����µ�λ�ã����¶���������ƿ������ˮϴ���Ӽ�ʽ�ζ����з���20.00mL������Һ����ƿ�У�
�ڽ���ʽ�ζ���������ˮϴ�������ñ���Һ��ϴ2-3�κ�������ע��0.1000mol-L-1�����ᣬ���ڵζ��ܵļ��첿�ֳ�����Һ����ʹҺ�洦�ڡ�0���̶����µ�λ�ã����¶�����
������ƿ�е���ָʾ�������еζ����ζ����յ㣬��¼���ݣ�
���ظ����Ϲ���2�Σ��Իش��������⣺
��1��Ӧ��NaOH��Һע��ͼ1-�е�______��ѡ��ס����ҡ����У�
��2����С���ڲ�����еĴ�����______���ɴ���ɵIJⶨ���______��ƫ�ߡ�ƫ�ͻ���Ӱ�죩��
��3����ͼ2��ij�εζ�ʱ�ĵζ����е�Һ�棬ͼ2��ʾ50mL�ζ�����Һ���λ�ã���A��C�̶ȼ����1mL��A���Ŀ̶�Ϊ25���ζ�����Һ�����ӦΪ______mL��
��4���õζ������Т�Ӧѡ�õ�ָʾ����______���ζ�ʱ�����ֿ��Ƶζ��ܻ����������ճ���ƿ���ߵα����۾�ע�ӣ�______�����ȷ���յ㣿______��
��5�������������ݣ�
| �ζ����� | ����Һ�����mL�� | �����������mL�� | |
| �ζ�ǰ������mL�� | �ζ��������mL�� | ||
| ��һ�� | 20.00 | 0.52 | 25.42 |
| �ڶ��� | 20.00 | 4.07 | 29.17 |
��6������1mol/L��0.1mol/L��HCl��Һ��Ӧ��______��HCl��Һ��ԭ����______��

��1����ͼ��֪��Ϊ��ʽ�ζ��ܣ���Ϊ��ʽ�ζ��ܣ�NaOH��ҺӦ�ü�ʽ�ζ��ܣ���ѡ����ע��NaOH��Һ���ʴ�Ϊ���ң�
��2���ɲ�����֪û����ϴ��ʽ�ζ��ܣ���ͬ���ʱ������ʵ������٣��������ĵ�����٣������NaOH��Ũ��ƫ�ͣ��ʴ�Ϊ��û���ô���NaOH��Һ��ϴ��ʽ�ζ��ܣ�ƫ�ͣ�
��3��A��C�̶ȼ����1mL��ÿ���̶�Ϊ0.1mL��A���Ŀ̶�Ϊ25��B�Ŀ̶ȱ�A����ͼ��֪�����4���̶ȣ���BΪ25.40���ʴ�Ϊ��25.40��
��4����ѡ���̪���۲쵽��ƿ����Һ����ɫ��dz��ɫ��Ϊ��ɫ���ﵽ�ζ��յ㣬��ѡ����ȣ��۲쵽��Һ�ɻ�ɫ��Ϊ��ɫ����ﵽ�ζ��յ㣬
�ʴ�Ϊ����̪������ȣ�����ƿ����Һ��ɫ�ı仯����̪Ϊָʾ��ʱdz��ɫ��Ϊ��ɫ�Ұ���Ӳ���ԭ�������Ϊָʾ��ʱ��ɫ��ɫ��ɫ�Ұ���Ӳ���ԭ����
��5����һ����Ϊ25.42-0.52=24.90mL���ڶ�����Ϊ29.17-4.07=25.10mL��������Ϊ
=25.00mL��
������к͵�ʵ�ʿ�֪��25.00mL��0.001L/mL��0.1000mol?L-1=20.00mL��0.001L/mL��c��������c���=0.1250mol/L���ʴ�Ϊ��0.1250mol/L��
��6��������Ũ�Ƚϴ�ʱ���ļ�������С�������ϴ���Ӧѡ��0.1mol/L��HCl��Һ���ʴ�Ϊ��0.1mol/L�������1mol/L����ζ������������ԼΪ2.50mL����������ϴ�
��2���ɲ�����֪û����ϴ��ʽ�ζ��ܣ���ͬ���ʱ������ʵ������٣��������ĵ�����٣������NaOH��Ũ��ƫ�ͣ��ʴ�Ϊ��û���ô���NaOH��Һ��ϴ��ʽ�ζ��ܣ�ƫ�ͣ�
��3��A��C�̶ȼ����1mL��ÿ���̶�Ϊ0.1mL��A���Ŀ̶�Ϊ25��B�Ŀ̶ȱ�A����ͼ��֪�����4���̶ȣ���BΪ25.40���ʴ�Ϊ��25.40��
��4����ѡ���̪���۲쵽��ƿ����Һ����ɫ��dz��ɫ��Ϊ��ɫ���ﵽ�ζ��յ㣬��ѡ����ȣ��۲쵽��Һ�ɻ�ɫ��Ϊ��ɫ����ﵽ�ζ��յ㣬
�ʴ�Ϊ����̪������ȣ�����ƿ����Һ��ɫ�ı仯����̪Ϊָʾ��ʱdz��ɫ��Ϊ��ɫ�Ұ���Ӳ���ԭ�������Ϊָʾ��ʱ��ɫ��ɫ��ɫ�Ұ���Ӳ���ԭ����
��5����һ����Ϊ25.42-0.52=24.90mL���ڶ�����Ϊ29.17-4.07=25.10mL��������Ϊ
| 24.90mL+25.10mL |
| 2 |
������к͵�ʵ�ʿ�֪��25.00mL��0.001L/mL��0.1000mol?L-1=20.00mL��0.001L/mL��c��������c���=0.1250mol/L���ʴ�Ϊ��0.1250mol/L��
��6��������Ũ�Ƚϴ�ʱ���ļ�������С�������ϴ���Ӧѡ��0.1mol/L��HCl��Һ���ʴ�Ϊ��0.1mol/L�������1mol/L����ζ������������ԼΪ2.50mL����������ϴ�

��ϰ��ϵ�д�
�����Ŀ
 ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£� ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£� ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�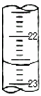
 ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�