��Ŀ����
 ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£��ٽ���ʽ�ζ���������ˮϴ�����ô�����Һ��ϴ2��3�κ���ע�������Һ�����ڵζ��ܵļ��첿�ֳ�����Һ����ʹ��Һ�洦�ڡ�0���̶����µ�λ�ã����¶�����
�ڽ���ƿ������ˮϴ����ֱ�ӴӼ�ʽ�ζ����з���20.00mL������Һ����ƿ�У�
�۽���ʽ�ζ���������ˮϴ��������������ע��0.1000mol/L��ϡ������Һ�����ڵζ��ܵļ��첿�ֳ�����Һ����ʹҺ�洦�ڡ�0���̶����µ�λ�ã����¶�����
������ƿ�е��뼸�η�̪��ָʾ�������еζ����ζ���ָʾ���պñ�ɫ���Ұ��������ɫ���ٸı�Ϊֹ�����������������ΪV1mL��
���ظ����Ϲ��̣����ڵζ�����������ƿ����5mL����ˮ���������ϡ����ΪV2mL��
�Իش��������⣺
��1����ƿ�е���Һ��
��
��
ɫ��Ϊ��
��
ɫʱ��ֹͣ�ζ�����2���ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲�
B
B
A���ζ�����Һ��ı仯�� B����ƿ����Һ��ɫ�ı仯
��3���������������д����һ����
��
��
������ţ����ɴ���ɵIJⶨ���ƫ��
ƫ��
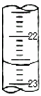
���ƫ�ߡ�����ƫ�͡�����Ӱ�족������4��ij�εζ�ʱ�ĵζ����е�Һ����ͼ���������Ϊ
13.60
13.60
mL������¼����ʱ����ʼʱ���ӣ��յ�ʱ���ӣ���������ҺŨ�Ȼ�ƫ��
ƫ��
���ƫ�ߡ�����ƫ�͡�����Ӱ�족������5�������������ݣ�
| �ζ����� | ����Һ�����mL�� | �����������mL�� | |
| �ζ�ǰ������mL�� | �ζ��������mL�� | ||
| ��һ�� | 20.00 | 0.50 | 25.45 |
| �ڶ��� | 20.00 | 4.00 | 29.05 |
| ������ | 20.00 | 3.00 | 30.00 |
| ���Ĵ� | 20.00 | 2.00 | 27.00 |
0.2500
0.2500
mol/L����������1�����ݵζ��յ㣬��ƿ�е���Һ�Ӻ�ɫ��Ϊ��ɫʱ���Ұ�����ڲ���ɫ��Ӧֹͣ�ζ���
��2�����ݵζ�ʱ�۾�Ӧ�۲���ƿ����Һ��ɫ�ı仯��
��3��������ʽ�ζ���������ˮϴ����Ӧ�ñ�Һ��ϴ������c�����⣩=
������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��4�����ݵζ��ܵĽṹ�;�ȷ��Ϊ0.01mL������������c�����⣩=
������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��5���ȷ������������Һ���������Ч�ԣ�Ȼ��������������Һ�����ƽ��ֵ��Ȼ��Ȼ����ݹ�ϵʽHCl��NaOH�����
��2�����ݵζ�ʱ�۾�Ӧ�۲���ƿ����Һ��ɫ�ı仯��
��3��������ʽ�ζ���������ˮϴ����Ӧ�ñ�Һ��ϴ������c�����⣩=
| 2��V(��)��c(��) |
| V(����) |
��4�����ݵζ��ܵĽṹ�;�ȷ��Ϊ0.01mL������������c�����⣩=
| 2��V(��)��c(��) |
| V(����) |
��5���ȷ������������Һ���������Ч�ԣ�Ȼ��������������Һ�����ƽ��ֵ��Ȼ��Ȼ����ݹ�ϵʽHCl��NaOH�����
����⣺��1����ζ��յ�ʱ����ƿ�е���Һ�Ӻ�ɫ��Ϊ��ɫʱ���Ұ�����ڲ���ɫ��ֹͣ�ζ����ʴ�Ϊ���죻�ޣ�
��2���ζ�ʱ�۾�Ӧ�۲���ƿ����Һ��ɫ�ı仯�����ж��յ�ĵ����ѡ��B��
��3����ʽ�ζ���������ˮϴ����Ӧ�ñ�Һ��ϴ�������������������д����һ���Ǣۣ�����ʽ�ζ���������ˮϴ��������������ע��0.1000mol/L��ϡ������Һ����ϡ������Һ��Ũ��ƫС������Һ�����ʵ������䣬�������ı�Һ�����ƫ����c�����⣩=
��֪��c�����⣩ƫ�ߣ�
��ѡ���ۣ�ƫ�ߣ�
��4���ζ����е�Һ�����Ϊ13.60 mL������¼����ʱ����ʼʱ���ӣ��յ�ʱ���ӣ���V������ƫ�ͣ�c�����⣩=
��֪��c�����⣩ƫ�ͣ�
�ʴ�Ϊ��13.60��ƫ�ͣ�
��5�����������Һ������ֱ�Ϊ��24.95mL��25.05mL��27.00mL��25.00mL��������������Ч�������Һ��ƽ�����Ϊ
=25.00mL��
H2SO4 ��2NaOH
1 2
0.1000mol/L��25.00mL C��NaOH����20mL
=
��
���c��NaOH��=0.2500mol/L��
�ʴ�Ϊ��0.2500��
��2���ζ�ʱ�۾�Ӧ�۲���ƿ����Һ��ɫ�ı仯�����ж��յ�ĵ����ѡ��B��
��3����ʽ�ζ���������ˮϴ����Ӧ�ñ�Һ��ϴ�������������������д����һ���Ǣۣ�����ʽ�ζ���������ˮϴ��������������ע��0.1000mol/L��ϡ������Һ����ϡ������Һ��Ũ��ƫС������Һ�����ʵ������䣬�������ı�Һ�����ƫ����c�����⣩=
| 2��V(��)��c(��) |
| V(����) |
��ѡ���ۣ�ƫ�ߣ�
��4���ζ����е�Һ�����Ϊ13.60 mL������¼����ʱ����ʼʱ���ӣ��յ�ʱ���ӣ���V������ƫ�ͣ�c�����⣩=
| 2��V(��)��c(��) |
| V(����) |
�ʴ�Ϊ��13.60��ƫ�ͣ�
��5�����������Һ������ֱ�Ϊ��24.95mL��25.05mL��27.00mL��25.00mL��������������Ч�������Һ��ƽ�����Ϊ
| 24.95mL+25.05mL+25.00mL |
| 3 |
H2SO4 ��2NaOH
1 2
0.1000mol/L��25.00mL C��NaOH����20mL
| 1 |
| 0.1000mol/L��25.00mL |
| 2 |
| C(NaOH)��20mL |
���c��NaOH��=0.2500mol/L��
�ʴ�Ϊ��0.2500��
������������Ҫ����������к͵ζ��IJ������������Լ��йصĻ�ѧ���㣬�Ѷ��еȣ������к͵ζ���ԭ���ǽ���Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
 ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£� ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£� ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�