��Ŀ����
 ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£��ٽ���ʽ�ζ���������ˮϴ����������Һ��ϴ����ע�������Һ�����ڵζ��ܵļ��첿�ֳ�����Һ����ʹҺ�洦�ڡ�0���̶����µ�λ�ã����¶���������ƿ������ˮϴ�����ô�����Һ��ϴ��ƿ2��3�Σ��Ӽ�ʽ�ζ����з���20.00mL������Һ����ƿ�У�
�ڽ���ʽ�ζ���������ˮϴ�������ñ���Һ��ϴ2-3�κ�������ע��0.1000mol?L-1�����ᣬ���ڵζ��ܵļ��첿�ֳ�����Һ����ʹҺ�洦�ڡ�0���̶����µ�λ�ã����¶�����
������ƿ�е����̪��ָʾ�������еζ����ζ���ָʾ���պñ�ɫ���Ұ��������ɫ���ٸı�Ϊֹ�����������������ΪV1mL��
���ظ����Ϲ��̣����ڵζ�����������ƿ����5mL������ˮ�����������������ΪV2mL��
�Իش��������⣺
��1����ƿ�е���Һ��
��
��
ɫ��Ϊ��
��
ɫʱ��ֹͣ�ζ�����2����С���ڲ�����еĴ�����
��ƿ�����ô���Һ��ϴ
��ƿ�����ô���Һ��ϴ
���ɴ���ɵIJⶨ���ƫ��
ƫ��
��ƫ�ߡ�ƫ�ͻ���Ӱ�죩����3����ͼ����ij�εζ�ʱ�ĵζ����е�Һ�棬�����Ϊ
22.60
22.60
mL����4�������������ݣ�
| �ζ����� | ����Һ�����mL�� | �����������mL�� | |
| �ζ�ǰ������mL�� | �ζ��������mL�� | ||
| ��һ�� | 20.00 | 0.50 | 25.40 |
| �ڶ��� | 20.00 | 4.00 | 29.10 |
0.1250
0.1250
mol?L-1����������1�����ݱ���ζ�NaOH��Һ���Է�̪��ָʾ�����ζ��յ���ɫ�ı仯��
��2��������ƿװҺǰ������ϴ�����ݸ���c���=
�жϲ��������������������Ӱ�죻
��3�����ݵζ��ܵĽṹ�;�ȷ�ȣ�
��3�����ж����ݵĺ����ԣ�Ȼ��������Һ��ƽ�������Ȼ�����HCl��NaOH��NaOH��Һ�����ʵ���Ũ�ȣ�
��2��������ƿװҺǰ������ϴ�����ݸ���c���=
| c(��)��V(��) |
| V(��) |
��3�����ݵζ��ܵĽṹ�;�ȷ�ȣ�
��3�����ж����ݵĺ����ԣ�Ȼ��������Һ��ƽ�������Ȼ�����HCl��NaOH��NaOH��Һ�����ʵ���Ũ�ȣ�
����⣺��1������ζ�NaOH��Һ���Է�̪��ָʾ�����ζ��յ�ʱ����Һ����ɫ�ɺ�ɫ��Ϊ��ɫ���ʴ�Ϊ���죻
��2����ƿװҺǰ������ϴ������ƿ�ô���Һ��ϴ������Һ�����ʵ���ƫ�࣬���V���ᣩ������c���=
��֪��c���ƫ�ߣ��ʴ�Ϊ����ƿ�����ô���Һ��ϴ��ƫ�ߣ�
��3���ζ����е�Һ��Ķ���Ϊ22.60mL���ʴ�Ϊ��22.60��
��4���������ĵ������Һ�����Ϊ24.90mL��25.10mL������Ч��������Һ��ƽ�����Ϊ
=
25.00mL��
HCl��NaOH
1 1
0.1000mol?L-1��25.00 c���ռ��20.00
���c�����ᣩ=0.1250mol/L���ʴ�Ϊ��0.1250��
��2����ƿװҺǰ������ϴ������ƿ�ô���Һ��ϴ������Һ�����ʵ���ƫ�࣬���V���ᣩ������c���=
| c(��)��V(��) |
| V(��) |
��3���ζ����е�Һ��Ķ���Ϊ22.60mL���ʴ�Ϊ��22.60��
��4���������ĵ������Һ�����Ϊ24.90mL��25.10mL������Ч��������Һ��ƽ�����Ϊ
| 24.90mL+25.10mL |
| 2 |
25.00mL��
HCl��NaOH
1 1
0.1000mol?L-1��25.00 c���ռ��20.00
���c�����ᣩ=0.1250mol/L���ʴ�Ϊ��0.1250��
���������⿼���к͵ζ��IJ����Լ�ע�������������ʱ��������һ�����͵��������Թ��Ϊ�Ա���Һ�����Ӱ�죬�����Һ�����ƫС����ô��õ����ʵ�����Ũ��ҲƫС�������Һ�����ƫ����ô��õ����ʵ�����Ũ��Ҳƫ��

��ϰ��ϵ�д�
�����Ŀ
 ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£� ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�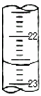
 ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�