��Ŀ����
18��I���Ҵ��е���78�棬����ˮ������������ܣ����ѵķе�Ϊ34.6�棬������ˮ���ڱ���Na2CO3��Һ�м������ܣ����Ѽ���ȼ�գ�ʵ���������ѵķ�Ӧԭ���ǣ�2CH3CH2OH$��_{��}^{ŨH_{2}SO_{4}}$CH3CH2OCH2CH3+H2O
��1����Ӧ��Ӧ�����ʯ���������Ƿ�ֹ�ٷУ�
��2����Ӧ���¶ȼƵ�λ���Dz��뷴Ӧ��Һ�����£������ܴ���ƿ�ף�
��3����װ���Ƶ������п��ܺ��д����ĸ�������ϩ����ѧ����ʽ��CH3CH2OH$��_{170��}^{ŨH_{2}SO_{4}}$CH2=CH2��+H2O��
II�����ͼ������������Ĵ������ʣ��ش��������⣺
��1�����ڳ��³�ѹ�³�Һ̬��
��2�����ͼ����к�̼���ϸߵ��DZ���
��3��д��������������ȼ�յĻ�ѧ����ʽCH4+2O2$\stackrel{��ȼ}{��}$CO2+2H2O�����������ʵ����ı��ͼ���ֱ�ȼ�գ��������ϴ���DZ���
��4��д�������嵥�ʷ�Ӧ�Ļ�ѧ����ʽ
 ��
��
���� ��1��ΪҺ����ȼӷ�ʯ���Ƿ�ֹҺ���ٷУ�
��2����Ӧ����Ҫ���Ʒ�Ӧ�¶���140�棬�¶ȼ���Ҫ�������Һ���¶ȣ�
��3���Ҵ���Ũ���������¼��ȵ�170��Ϳ���������ϩ��
��1�����ڳ��³�ѹ�³�Һ̬��
��2�����������ʽ�жϺ�̼���ߵͣ�
��3������ȼ�����ɶ�����̼��ˮ�����ݷ���ʽ�ж���������������С��
��4�����廯�������������£�����Һ�巢��ȡ����Ӧ��
��� �⣺I����1����Ӧ��Ӧ�����ʯ���������Ƿ�ֹҺ���ٷУ�
�ʴ�Ϊ����ֹ�ٷУ�
��2���¶ȼƲ������ǻ��Һ���¶ȣ������¶ȼ�ˮ����Ӧ�ò��뷴Ӧ��Һ�����£������ܴ���ƿ�ף�
�ʴ�Ϊ�����뷴Ӧ��Һ�����£������ܴ���ƿ�ף�
��3�����Ҵ���Ũ���������¼��ȵ�170��Ϳ���������ϩ����ѧ����ʽΪ��CH3CH2OH$��_{170��}^{ŨH_{2}SO_{4}}$CH2=CH2��+H2O��
�ʴ�Ϊ��CH3CH2OH$��_{170��}^{ŨH_{2}SO_{4}}$CH2=CH2��+H2O��
II����1�����ڳ��³�ѹ�³�Һ̬��
�ʴ�Ϊ��Һ��
��2�����������ʽ�жϺ�̼���ߵͣ�����ķ���ʽ�����ʽ����CH4���������ʽΪCH�����к�̼���ߣ�
�ʴ�Ϊ������
��3������ȼ�����ɶ�����̼��ˮ����Ӧ����ʽΪ��CH4+2O2$\stackrel{��ȼ}{��}$CO2+2H2O����ȼ�շ���ʽΪ��C6H6+7.5O2$\stackrel{��ȼ}{��}$6 CO2+3H2O�������ʵ����ı��ͼ���ֱ�ȼ�գ��������ϴ���DZ���
�ʴ�Ϊ��CH4+2O2$\stackrel{��ȼ}{��}$CO2+2H2O������
��4�����廯�������������������£�����Һ�巢��ȡ����Ӧ����Ӧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼�����Ҵ��Ʊ����ѣ��漰��Ӧ�����Ŀ��ƶ�ʵ���Ӱ�죬�������˼���ͱ������ʣ��ѶȽ�С���Ƚ����к�̼�����ɸ���̼������жϣ���ֵԽ����̼��Խ�ߣ���Ŀ�ѶȲ���

 ��У����ϵ�д�
��У����ϵ�д�| A�� | Ũ������ձ�ƣ�֤������ȶ����Ҳ����к���ɫ���������Ũ���� | |
| B�� | ��ij��Һ�м����Ȼ�����Һ��ϡ���ᣬ���ɰ�ɫ��������ԭ��Һһ����������� | |
| C�� | �����£�����Ƭ����Ũ����������������˵��������Ũ���ᷴӦ | |
| D�� | ��ˮ��ͨ��SO2����ɫ��ȥ��˵��SO2������Ư���� |
| A�� | ���Ҵ���ȡ��ˮ�е��嵥�ʿ�ѡ�÷�Һ©�� | |
| B�� | Ϊ�˱�֤����Ч�����������ʱ����ˮ����Ҫ������ˮ����������ͬ | |
| C�� | �������ʱ�¶ȼƵ�ˮ����Ҫ��Բ����ƿ��֧�ܿ���ƽ | |
| D�� | ��Һʱ���轫��Һ©���Ͽڵ����Ӵ�ʹ�������ۺ�С����ͨ |
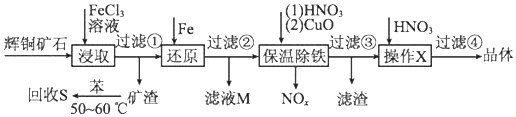
��֪����������������������ʽ��ʼ��������ȫ����ʱ��Һ��pH���
| ������ | Cu��OH��2 | Fe��OH��3 | Fe��OH��2 |
| ��ʼ����pH | 4.7 | 2.7 | 7.6 |
| ��ȫ����pH | 6.7 | 3.7 | 9.6 |
��2������S�������¶ȿ���50�桫60��֮�䣬���˹�����͵�ԭ�����¶ȸ߱����ӷ����¶ȵ��ܽ�����С��
��3�����³��������м���CuO��Ŀ���ǵ�����Һ��pH��ʹFe3+��ȫת��ΪFe��OH��3������
��4�����ˢ����õ�����Һ���������������X�����ˢܵõ�����ͭ���壮����X������Ũ������ȴ�ᾧ����������ƣ���
| A�� | ��ˮ����ɫ��dz����ɫ������Ϊ��ˮ�к���Cl2 | |
| B�� | ������ˮ��ʹ��ɫ������ɫ��˵����ˮ�к�HClO | |
| C�� | ����ˮ�м���NaHCO3��ĩ�������ݲ�����˵����ˮ�к���H+ | |
| D�� | ����̿����ˮ������Ư�����ã���Ư�Ļ�ѧԭ����ͬ |
| A�� | 2-������ | B�� | ������ | ||
| C�� | 2��2��3��3-�ļ����� | D�� | 3-������ |
| A�� | 18gH2O����������Ϊ10NA | |
| B�� | ���³�ѹ�£�48gO3��O2����ԭ����Ŀ3NA | |
| C�� | 1molNa2O2������������ĿΪNA | |
| D�� | 11.2L����������ԭ����ΪNA |
 ��֪���±�Ϊ25��ʱijЩ����ĵ���ƽ�ⳣ����
��֪���±�Ϊ25��ʱijЩ����ĵ���ƽ�ⳣ����| CH3COOH | HClO | H2CO3 |
| Ka=1.8��10-5 | Ka=3.0��10-8 | Ka1=4.4��10-7Ka2=4.7��10-11 |
| A�� | 25��ʱ����ͬŨ�ȵ�NaHCO3��Һ�ļ��Դ���NaClO��Һ | |
| B�� | ͼ����a��ĵ�������С��c�� | |
| C�� | ͼ�� I����CH3COOH��Һ��ϡ�� | |
| D�� | ͼ����a�������Ũ�ȴ���b�������Ũ�� |
