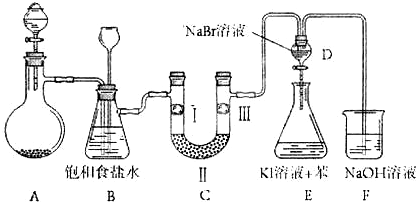
��Ŀ����
��ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ�ã��г��豸���ԣ���
��1���Ʊ�����ѡ�õ�ҩƷΪ��Ư�۹����Ũ���ᣬ��صĻ�ѧ��Ӧ����ʽΪ��___________________________________��
��2��װ��B�б���ʳ��ˮ��������_______��ͬʱװ��B���ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������д����������ʱB�е�����______________________________��
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C�Т����η���__________��
| | a | b | c | d |
| �� | �������ɫ���� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
| �� | ��ʯ�� | �轺 | Ũ���� | ��ˮ�Ȼ��� |
| �� | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
��5����������װ��D��������Һ����װ��E�У����۲쵽��������__________��
��6��װ��F��������__________�����������F�е�ҩƷ�ɸ���������NaHSO
 ��Һ���жϸ���NaHSO
��Һ���жϸ���NaHSO ��Һ�Ƿ����__________����ǡ���������𡰷������ӷ���ʽ����ԭ��____________________�����ǡ����ý��ͣ���
��Һ�Ƿ����__________����ǡ���������𡰷������ӷ���ʽ����ԭ��____________________�����ǡ����ý��ͣ���
��1��Ca(ClO) +4HCl(Ũ)=CaCl
+4HCl(Ũ)=CaCl +2Cl
+2Cl ��+2H
��+2H O��
O��
��2����ȥCl �е�HCl��B�г���©����Һ���������γ�ˮ����
�е�HCl��B�г���©����Һ���������γ�ˮ����
��3��d�� ��4���ƣ� ��5��E����Һ��Ϊ���㣬�ϲ㣨���㣩Ϊ�Ϻ�ɫ��
��6������δ��Ӧ����������HSO3-+Cl2+H2O=SO42-+2Cl-+3H+ HSO3-+H+=SO2��+H2O
���������������1��Ư�۾�����Ҫ�ɷ���Ca(ClO)2����ǿ�����ԣ���Ũ���ᷴӦ�ķ���ʽΪCa(ClO)2 + 4HCl(Ũ)= CaCl2 + 2Cl2��+ 2H2O��
��2�����ɵ������л����Ȼ������ͨ������ʳ��ˮ��ȥ����C�ж�������B��ѹǿ������ˮ����ѹ���Ӷ��γ�һ��ˮ����
��3��Ҫ����������Ư���ԣ�����������ɫ��������Ϊ��ȡ����������ˮ�����������Ҫ����������ѡ�ù轺����ˮ�Ȼ��ƣ�������ѡ���ʯ�Һ�Ũ���ᡣIIIһ���Ǹ������ɫ������������ȷ��ѡ����d��
��4����������ˮ���Ի�ɫ��
��5�������л��ܼ������Ϻ�ɫ����Ϊ�����ܶ�С��ˮ�ģ����ϲ����Ϻ�ɫ��
��6�������ж�����Ⱦ����������Fװ�õ����������ն����������HSO3�����л�ԭ�ԣ��ɻ�ԭ����������ʽΪHSO3�� + Cl2 + H2O �� SO42�� + 2Cl�� + 3H+����Ϊ��Ӧ����Һ�����ԣ����Ծ��п������ɶ���������Ⱦ���������Բ���ѡ�á�
���㣺��������ʵ��
�����������ۺ��Խ�ǿ����Ҫ����ѧ���Ļ���֪ʶ���ѶȲ���

 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д���10�֣���ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ�ã��г��豸���ԣ���

��1���Ʊ�����ѡ�õ�ҩƷΪ��Ư�۾������Ũ���ᣬ��صĻ�ѧ��Ӧ����ʽΪ��
��
��2��װ��B�б���ʳ��ˮ�������� ��ͬʱװ��B���ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������д����������ʱB�е����� ��
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C��I��II��III���η��� ��
|
|
a |
b |
c |
d |
|
I |
�������ɫ���� |
�������ɫ���� |
ʪ�����ɫ���� |
ʪ�����ɫ���� |
|
II |
��ʯ�� |
�轺 |
Ũ���� |
��ˮ�Ȼ��� |
|
III |
ʪ�����ɫ���� |
ʪ�����ɫ���� |
�������ɫ���� |
�������ɫ���� |
��4�����װ��D��E��Ŀ���DZȽ��ȡ��塢��ķǽ����ԡ�����D�л���ͨ����������ʱ�����Կ�����ɫ��Һ��Ϊ���غ�ɫ��˵���ȵķǽ����Դ����塣��������װ��D��������Һ����װ��E�У����۲쵽�������� ��������
����ܡ����ܡ���˵����ķǽ�����ǿ�ڵ⣬ԭ���� ��