��Ŀ����
9��ú���ۺ����ö��ڸ��ƴ�����������Դ�����������Ҫ�����壮��1������˵����ȷ����abc������ţ���
a��ú�����л��������������ɵĸ��ӻ����
b��ú��ȼ�չ����л�����һ����̼����������������̳����к�����
c����������ʯ�ҡ���ʯ�ҡ�ʯ��ʯ�ȹ����ʹú��ȼ�չ����������ȶ���������
d��ú�ĸ������ڻ�ѧ�仯��ú��������Һ�������������仯
��2��ú�����������в������к�����H2S�ð�ˮ�����������ε����ӷ���ʽ��H2S+2NH3•H2O�T2NH4++S2-+2H2O��
��3��ȼú������һ������-���һʯ��һʯ�෨����ͼ1���£�

�ٳ����£���NaOH��Һ����SO2�õ�pH=6��NaHSO3��Һ������Һ������Ũ�ȵĴ�С˳����c��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-����
������������NaHSO3��Ӧ�Ļ�ѧ����ʽ��Ca��OH��2+NaHSO3�TCaSO3��+NaOH+H2O��
��4��ú�ļ��Һ�����ϳɶ����ѵ�������Ӧ���£�
��.2H2��g��+CO��g��?CH3OH��g����H=-90.8kJ•mol-1
��.2CH3OH��g��?CH3OCH3��g��+H2O��g����H=-23.5kJ•mol-1
��CO��g��+H2O��g��?CO2��g��+H2��g����H=-41.3kJ•mol-1
���ܷ�Ӧ�Ȼ�ѧ����ʽ��3H2��g��+3CO��g��?CH3OCH3��g��+CO2��g����H=-246.4 kJ•mol-1��
��Y��Y1��Y2����X�ɷֱ����ѹǿ���¶ȣ���ͼ2��ʾYһ��ʱ���ܷ�Ӧ��CO��ƽ��ת������X�ı仯��ϵ���ж�Y1��Y2�Ĵ�С��ϵ�����������ɣ�Y1��Y2����3H2��g��+3CO��g��?CH3OCH3��g��+CO2��g����֪��ѹǿ����CO��ƽ��ת���������¶����ߣ�CO��ƽ��ת���ʼ�С������
X����ѹǿ��Y�����¶ȣ�ѹǿһ��ʱ���¶�Խ��ƽ��ת����Խ��
���� ��1��a��ú�����л��������������ɵĸ��ӵĻ���
b����ú�к��е�Ԫ�غͲ���ȫȼ�շ�����
c��̼������ȷֽ�Ϊ�����ƣ��������������������������������������Ӧ��
d��ú�ĸ���ú��������Һ�������ڻ�ѧ�仯��
��2��H2S�ð�ˮ��������������狀�ˮ��
��3���ٳ����£�NaHSO3��Һ��pH=6��NaHSO3��Һ�����ԣ�˵������̶ȴ���ˮ��̶ȣ�
������������NaHSO3��Ӧ���ɳ���������ơ�NaOH��ˮ��
��4���پݸ�˹��������֪���Ȼ�ѧ����ʽ������Ӧ����ֵ���мӼ���������Ŀ���Ȼ�ѧ����ʽ����Ӧ��Ҳ������Ӧ����ֵ���мӼ���
��3H2��g��+3CO��g��?CH3OCH3��g��+CO2��g����H��0������Ӧ���������С��ͨ�����������¶Ⱥ�����ѹǿ����ƽ����ƶ�����ͨ��CO��ƽ��ת���ʵı仯�ж�X��Y��
��� �⣺��1��a��ú�����л��������������ɵĸ��ӵĻ�����Ҫ����CԪ�أ���a��ȷ��
b��ú�а�����Ԫ�غ͵�Ԫ�أ�����ȫȼ��ʱ����һ����̼����������������̳����к����ʣ���b��ȷ��
c��̼������ȷֽ�Ϊ�����ƣ�������������������������������ơ������Ʒ�����Ӧ����������ƣ�Ȼ���������������ȶ��������Σ���c��ȷ��
d��ú�ĸ���ú��������Һ�������ڻ�ѧ�仯����d����
�ʴ�Ϊ��abc��
��2��H2S�ð�ˮ��������������狀�ˮ�����ӷ���ʽΪ��H2S+2NH3•H2O�T2NH4++S2-+2H2O��
�ʴ�Ϊ��H2S+2NH3•H2O�T2NH4++S2-+2H2O��
��3���ٳ����£�NaHSO3��Һ��pH=6��NaHSO3��Һ�����ԣ�NaHSO3��Һ�д��������������ˮ������룬ˮ�����ʼ��ԣ����뵼��������ԣ��ݴ˷���������̶ȴ���ˮ��̶ȣ�������Ũ�ȴ�СΪ��c��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-����
�ʴ�Ϊ��c��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-����
������������NaHSO3��Ӧ���ɳ���������ơ�NaOH��ˮ����ѧ����ʽΪCa��OH��2+NaHSO3�TCaSO3��+NaOH+H2O��
�ʴ�Ϊ��Ca��OH��2+NaHSO3�TCaSO3��+NaOH+H2O��
��4���٣���2H2��g��+CO��g��?CH3OH��g����H=-90.8kJ•mol-1
��2CH3OH��g��?CH3OCH3��g��+H2O��g����H=-23.5kJ•mol-1
��CO��g��+H2O��g��?CO2��g��+H2��g����H=-41.3kJ•mol-1
�ɸ�˹���ɢ�+��+�١�2�õ�3H2��g��+3CO��g��?CH3OCH3��g��+CO2��g����H=-246.4 kJ•mol-1��
�ʴ�Ϊ��-246.4 kJ•mol-1��
��3H2��g��+3CO��g��?CH3OCH3��g��+CO2��g����H��0������Ӧ���������С�������¶ȣ�ƽ�������ƶ���CO��ƽ��ת���ʼ�С������ѹǿ��ƽ�������ƶ���
CO��ƽ��ת�����������X����ѹǿ��Y�����¶ȣ���ѹǿ�����£��¶�Խ�ߣ�CO��ƽ��ת����ԽС�����Y1��Y2��
�ʴ�Ϊ��Y1��Y2����3H2��g��+3CO��g��?CH3OCH3��g��+CO2��g����֪��ѹǿ����CO��ƽ��ת���������¶����ߣ�CO��ƽ��ת���ʼ�С������X����ѹǿ��Y�����¶ȣ�ѹǿһ��ʱ���¶�Խ��ƽ��ת����Խ��
���� ���⿼���Ϊ�ۺϣ��漰ú���ۺ����á���ѧ�����ӣ�����ʽ����д������Ũ�ȵĴ�С�Ƚϡ���Ӧ�ȵļ���ͻ�ѧƽ����ƶ�����Ŀ�Ѷ��еȣ����յ���ƽ�⡢ˮ��ƽ��Ȼ�ѧƽ�⼰������Ϊ���Ĺؼ�����Ŀ�Ѷ��еȣ�

| A�� | $\frac{1}{14}$mol/L | B�� | $\frac{4}{5}$mol/L | C�� | $\frac{1}{28}$mol/L | D�� | $\frac{1}{42}$mol/L |
| A�� | pH=3�������У���c��H+����pH=1�������е�3�� | |
| B�� | 1mol/L0.5L�� AlCl3��Һ�У������ӵ����ʵ�������0.5mol | |
| C�� | ͬ�¶�ͬ���ʵ���Ũ��ʱ��HF��HCN���룬��NaF��Һ�� pH �� NaCN ��Һ�� | |
| D�� | ����ˮ�������c��H+��=1��10-12mol/L����Һ�У�K+��ClO-��SO42-һ���ܴ������� |
| A�� | Na+��I-��CO${\;}_{3}^{2-}$��ClO- | B�� | Fe2+��H+��K+��NO${\;}_{3}^{-}$ | ||
| C�� | Ba2+��Na+��SCN-��Cl- | D�� | Cu2+��Fe2+��Cl-��NO${\;}_{3}^{-}$ |
| �� ���� | IA | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� | �� | |||
| 4 | �� | �� | �� |
��2���١��ۡ��ݵ��������������Ӧ��ˮ�����У�������ǿ����NaOH���ѧʽ����
��3���ڡ��ۡ�������Ԫ�ؿ��γɵ����ӣ����Ӱ뾶�ɴ�С��˳��ΪK+��Ca2+��Mg2+�������ӷ��ţ���
��4���ٺ͢���Ԫ���γɻ�����Ļ�ѧʽΪNaBr���û���������ʱ����ɫΪ
��ɫ���û��������Һ��Ԫ�آ�ĵ��ʷ�Ӧ�����ӷ���ʽΪCl2+2Br-=2Cl-+Br2
��5���õ���ʽ��ʾ��������Ԫ���н�������ǿ��Ԫ�غͷǽ�������ǿ��Ԫ���γɻ�����Ĺ���
 ��
�� | A�� | ��һ����ǿ�� | B�� | ��һ�������� | C�� | ��һ���Ƕ�Ԫ�� | D�� | ��һ����һԪ�� |
| A�� |  �� �� | B�� | ���͡� | ||
| C�� | CH3COCH3 �� CH3CHO | D�� | CH3Cl ��ClCH2CH2Cl |
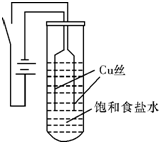
| ���� | �Ȼ�ͭ | ������ͭ | ��������ͭ �����ȶ��� | �Ȼ���ͭ |
| ��ɫ | �������ɫ��Ũ��Һ����ɫ��ϡ��Һ����ɫ | ��ɫ | �Ȼ�ɫ | ��ɫ |
| A�� | ���������������������ƶ� | B�� | ��ʼʱ����Cu �ŵ�����Cu2+ | ||
| C�� | ������ӦΪ2H2O+2e-=H2��+2OH- | D�� | ����ҺpH����CuCl��ת��ΪCuOH |
ʵ�����ƣ�±�ص��ʵ�������ǿ���Ƚ�
| ʵ�鲽�� | ʵ����� �����Դ�ǿ������˳���ȡ��塢�� |
| ����ˮ+1mL CCl4�������ã��۲����Ȼ�̼����ɫ | |
| ��NaBr��Һ+��ˮ+1mL CCl4�������ã��۲����Ȼ�̼����ɫ | |
| ��KI��Һ+��ˮ+1mL CCl4�������ã��۲����Ȼ�̼����ɫ |
ʵ��������£���ش�
��1����ɸ�ʵ�����õ���ʵ���������Թܡ���ͷ�ιܣ�
��2�����з�Ӧ�Ļ�ѧ����ʽΪ2NaBr+Cl2�T2NaCl+Br2��
���з�Ӧ�����ӷ���ʽΪCl2+2I-�T2Cl-+I2��
��3��CCl4��ʵ�����������������ȡ����
��4����ͬѧ��ʵ����Ʋ���֮����û�бȽ�Br2��I2��������ǿ�����Ľ��İ취�ǽ���ˮ����KI������ֽ�ϣ��۲���ֽ�Ƿ����ɫ����KI��Һ+��ˮ+1 mL CCl4�������ã��۲����Ȼ�̼����ɫ����