��Ŀ����
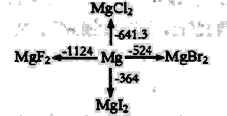
����Ŀ����������ĵ���ƽ�ⳣ�������
���� | HCOOH | HClO | H2CO3 | H2SO3 |
����ƽ�� ������25�棩 | Ka��1.77��10��4 | Ka��4.0��10��8 | Ka1��4.3��10��7 Ka2��4.7��10��11 | Ka1��1.54��10��2 Ka2��1.02��10��7 |
��1�������¢�0.1mol��L-1HCOONa����0.1mol��L-1NaClO����0.1mol��L-1Na2CO3����0.1mol��L-1NaHCO3��Һ��pH�ɴ�С�Ĺ�ϵΪ_______________________��
��2��Ũ�Ⱦ�Ϊ0.1 mol��L��1��Na2SO3��Na2CO3�Ļ����Һ�У�SO32-��CO32-��HSO3-��HCO3-Ũ�ȴӴ�С��˳��Ϊ_______________________��
��3���������ӷ���ʽ��ȷ����___________������ĸ����
A.2ClO-+H2O+CO2=2HClO+CO32-
B.2HCOOH+CO32-=2HCOO-+H2O+CO2��
C.H2SO3+=2HCOO-=2HCOOH+SO32-
D.Cl2+H2O+2CO32-=2HCO3-+Cl-+ClO-
��4��ij�¶ȣ�T�棩�µ���Һ�У�c��H+��=10-xmol��L-1��c��HO-��=10-ymol��L-1��x��y�Ĺ�ϵ��ͼ��ʾ��

�ٴ��¶��£�0.01mol/L��NaOH��Һ��ˮ�������OH-Ũ��Ϊ____________��
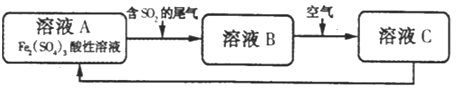
���ڴ��¶��£�0.1mol��L-1��NaHSO4��Һ��0.1mol��L-1��Ba��OH��2��Һ���±��мס��ҡ���������ͬ��ʽ��ϣ�
�� | �� | �� | �� | |
0.1mol��L-1��Ba��OH��2 | 10 | 10 | 10 | 10 |
0.1mol��L-1��NaHSO4 | 5 | 10 | 15 | 20 |
������ʽ��Ϻ�������Һ��____________���������������������������ԣ�д�����ҷ�ʽ��Ϻ�Ӧ�����ӷ���ʽ��______________������ʽ��Ϻ�������Һ��pHΪ____________��
���𰸡��ۣ��ڣ��ܣ��� c��SO32-����c��CO32-����c��HCO3-����c��HSO3-�� BD 1��10-10mol��L-1 �� Ba2����OH����H����SO42��=BaSO4����H2O 11
��������
��1���������ʶ���ǿ�������Σ���Һ�Լ��ԣ����ݱ������ݣ��Ƴ������H�������Ĵ�С��H2SO3>HCOOH>H2CO3>HSO3��>HClO>HCO3������������ˮ��Ĺ��ɣ��ó�pH�Ӵ�С�Ĺ�ϵΪNa2CO3>NaClO>Na2SO3>NaHCO3>HCOONa>NaHSO3�����Т�>��>��>�٣�
��2������ˮ��̶�Խ����Һ���������Ũ��ԽС������(1)�ķ������ó���Һ������Ũ�ȣ�c(SO32��)>c(CO32��)>c(HCO3��)>c(HSO3��)��
��3�����ݱ������ݣ��Ƴ������H�������Ĵ�С��H2SO3>HCOOH>H2CO3>HSO3��>HClO>HCO3����
A��HClO�����H������ǿ��HCO3������˷�Ӧ�����ӷ���ʽΪClO����H2O��CO2=HClO��HCO3������A����
B��HCOOH������ǿ��̼�ᣬ����2HCOOH��CO32��=2HCOO����H2O��CO2������B��ȷ��
C�����ݵ����H��ǿ�����Ƴ�H2SO3��HCOO��=HCOOH��HSO3������C����
D��Cl2����H2O��ӦCl2��H2O![]() HCl��HClO���п��ܷ���Cl2��H2O��2CO32��=2HCO3����Cl����ClO������D��ȷ��
HCl��HClO���п��ܷ���Cl2��H2O��2CO32��=2HCO3����Cl����ClO������D��ȷ��
��ѡBD��
��4���ٸ��¶��µ�ˮ�����ӻ�Ϊc(H��)��c(OH��)=100��10��12=10��12�����¶��£�0.01mol��L��1��NaOH��Һ��ˮ�������c(OH��)=c(H��)=![]() =
=![]() =10��10mol��L��1��
=10��10mol��L��1��
��10mL0.1mol��L��1Ba(OH)2��20mL0.1mol��L��1NaHSO4��ϣ������ķ�Ӧ����ʽΪBa(OH)2��H2SO4=2H2O��BaSO4����Na2SO4����Һ�����ԣ����ҷ�ʽ��ϣ������ӷ���ʽΪ��Ba2����OH����H����SO42��=BaSO4����H2O������ʽ��ϣ��������ӷ�Ӧ����ʽΪBa2����OH����H����SO42��=BaSO4����H2O����Ӧ����Һ��n(OH��)=10��10��3L��0.1mol��L��1��2��5��10��3L��0.1mol��L��1=1.5��10��3mol����c(OH��)=![]() =0.1mol��L��1��pHΪ11��
=0.1mol��L��1��pHΪ11��

 ��ʦ����ָ���ο�ʱϵ�д�
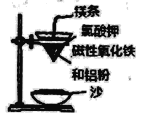
��ʦ����ָ���ο�ʱϵ�д�����Ŀ��ijʵ��С��ͬѧ��С��ľ̿�ھƾ������������ȣ�Ѹ��Ͷ���ȵ�Ũ�����У��������ҷ�Ӧ��ͬʱ�д�������ɫ���������Һ����ľ̿Ѹ��ȼ�շ������⡣���������Ͽ�֪Ũ����ֽ����NO2��O2��Ϊ��̽����Һ����ľ̿Ѹ��ȼ�շ�����������ԭ��С��ͬѧ����ͼװ���������ʵ�飺

��� | ʵ��I | ʵ��II |
����ƿ����ʢ���� | O2 | ����Ũ���� ���������� |
���� | ľ̿Ѹ��ȼ�շ������� | ľ̿Ѹ��ȼ�շ������� |
����˵����ȷ����
A. Ũ����ֽ������V(NO2):V(O2)=1:1
B. ����ɫ����IJ�������ľ̿��Ũ���ᷢ���˷�Ӧ
C. ��ʹҺ����ľ̿Ѹ��ȼ�շ����������Ҫ����������
D. ����ʵ�鲻��ȷ��ʹҺ����ľ̿Ѹ��ȼ�շ����������Ҫ����һ����NO2