��Ŀ����
 A��B��C��D��E��F��G��H�Ǻ˵������������Ķ���������Ԫ�أ�Ԫ��A��ԭ�Ӱ뾶������Ԫ������С�ģ�A��Eͬ���壬B��C��Dͬ���ڣ�D��G������������ȣ�G��������ΪD��2����Ԫ��B��һ�ֳ������ʿ������Ե缫���ϣ�������������Ϊ�����������壮B��D��G��������֮�͵���F��H��������֮�ͣ�I�������ճ��������������Ľ������ױ���ʴ�����ش��������⣺
A��B��C��D��E��F��G��H�Ǻ˵������������Ķ���������Ԫ�أ�Ԫ��A��ԭ�Ӱ뾶������Ԫ������С�ģ�A��Eͬ���壬B��C��Dͬ���ڣ�D��G������������ȣ�G��������ΪD��2����Ԫ��B��һ�ֳ������ʿ������Ե缫���ϣ�������������Ϊ�����������壮B��D��G��������֮�͵���F��H��������֮�ͣ�I�������ճ��������������Ľ������ױ���ʴ�����ش��������⣺��1��IԪ�������ڱ��е�λ��
��2��������ĽṹʽΪ
��3������������Ϣ������˵������ȷ����
A��A��B���γɶ��ֻ�����
B�����ȶ��ԣ�H2D��H2G
C��Ԫ��G������������Ӧˮ��������Ա�H����
D�������Ӱ뾶�Ĵ�С˳��r��D����r��E����r��F��
E���е㣺H2D��H2G
F��ͬ��ͬѹ�£���a L CA3��b L AHͨ��ˮ�У���������Һ��pH=7����a��b
��4�������£���ͬŨ��F��I�����ӵ���Һ�еμ�NaOH��Һ��F��I��Ԫ���Ⱥ������F��OH��n��ȫ������pH��4.7��I��OH��n��ȫ������pH��2.8����ksp�ϴ���ǣ�
��5������H��I��ɵ�ij�ֻ��������Һ���У�����ͭƬ����Һ��������Ϊ��ɫ�����ݲ���������ķ�Ӧԭ��������Ƶ�ԭ�����ͼ��ʾ���䷴Ӧ�������缫��ӦʽΪ
��6������ʯī�缫��⺬��0.04mol CuGD4��0.04mol EH�Ļ����Һ400mL������������������784mL�������ʱ����Һ��pH=
���㣺λ�ýṹ���ʵ����ϵӦ��
ר�⣺
������A��B��C��D��E��F��G��H�Ǻ˵������������Ķ���������Ԫ�أ�Ԫ��A��ԭ�Ӱ뾶������Ԫ������С�ģ���A��HԪ�أ�Ԫ��B��һ�ֳ������ʿ������Ե缫���ϣ�������������Ϊ�����������壬��B��CԪ�أ�����CO2��A��Eͬ���壬��Eԭ����������B������E��Na��B��C��Dͬ���ڣ����ڵڶ����ڣ�D��G������������ȣ�����ͬ���壬G��������ΪD��2������DΪOԪ�ء�GΪSԪ�أ�Hԭ������������HΪCl��Cԭ����������̼����֮�䣬��CΪNԪ�أ�B��D��G��������֮�͵���F��H��������֮�ͣ���F������Ϊ6+8+16-17=13����FΪAl��I�������ճ��������������Ľ������ױ���ʴ������IΪFe��
��1��IΪFeԪ�أ����ڵ������ڵڢ��壻
��2������CO2��������̼ԭ������ԭ��֮���γ�2�Թ��õ��Ӷԣ�
��3��A����Ԫ�ء�̼Ԫ�ؿ����γ�����
B���ǽ�����Խǿ���⻯��Խ�ȶ���
C���ǽ�����Խǿ����ۺ����������Խǿ��
D�����Ӳ�ṹ��ͬ���˵����Խ�����Ӱ뾶ԽС�����Ӳ�Խ�����Ӱ뾶Խ��
E��ˮ����֮����������������ΪҺ̬��������Ϊ���壻
F��ͬ��ͬѹ�£���a L NH3��b L HClͨ��ˮ�У���������Һ��pH=7�����ݵ���غ���Һ��c��NH4+��=c��Cl-���������غ��֪��c��NH4+��+c��c��NH3��H2O����c��Cl-�����ݴ��жϣ�
��4������ʱPHԽС��˵����������Խ�׳�����������ɽṹ��ͬ��Խ�׳��������ܶȻ�ԽС��
��5������Cl��Fe��ɵ�ij�ֻ��������Һ���У�����ͭƬ����Һ��������Ϊ��ɫ������ΪFeCl3������������ԭ��Ӧ�������ӻ�õ��������������ӣ�
��6������ʯī�缫��⺬��0.04mol CuSO4��0.04mol NaCl�Ļ����Һ400mL����������������Ӧ����������ȫ�ŵ�õ�����Ϊ0.02mol��ʵ����������Ϊ
=0.035mol������������Ϊ0.035mol-0.02mol=0.015mol��ת�Ƶ���Ϊ0.04mol+0.015mol��4=0.1mol����Һ��ͭ������ȫ�ŵ��õ���Ϊ0.08mol��С��0.1mol���ɵ���غ��жϵ�����ҺΪH2SO4��Na2SO4�����ݵ���غ����n��H+������������c��H+�����ٸ���pH=-lgc��H+�����㣮
��1��IΪFeԪ�أ����ڵ������ڵڢ��壻
��2������CO2��������̼ԭ������ԭ��֮���γ�2�Թ��õ��Ӷԣ�
��3��A����Ԫ�ء�̼Ԫ�ؿ����γ�����
B���ǽ�����Խǿ���⻯��Խ�ȶ���
C���ǽ�����Խǿ����ۺ����������Խǿ��
D�����Ӳ�ṹ��ͬ���˵����Խ�����Ӱ뾶ԽС�����Ӳ�Խ�����Ӱ뾶Խ��
E��ˮ����֮����������������ΪҺ̬��������Ϊ���壻
F��ͬ��ͬѹ�£���a L NH3��b L HClͨ��ˮ�У���������Һ��pH=7�����ݵ���غ���Һ��c��NH4+��=c��Cl-���������غ��֪��c��NH4+��+c��c��NH3��H2O����c��Cl-�����ݴ��жϣ�
��4������ʱPHԽС��˵����������Խ�׳�����������ɽṹ��ͬ��Խ�׳��������ܶȻ�ԽС��
��5������Cl��Fe��ɵ�ij�ֻ��������Һ���У�����ͭƬ����Һ��������Ϊ��ɫ������ΪFeCl3������������ԭ��Ӧ�������ӻ�õ��������������ӣ�
��6������ʯī�缫��⺬��0.04mol CuSO4��0.04mol NaCl�Ļ����Һ400mL����������������Ӧ����������ȫ�ŵ�õ�����Ϊ0.02mol��ʵ����������Ϊ
| 0.784L |
| 22.4L/mol |
���
�⣺A��B��C��D��E��F��G��H�Ǻ˵������������Ķ���������Ԫ�أ�Ԫ��A��ԭ�Ӱ뾶������Ԫ������С�ģ���A��HԪ�أ�Ԫ��B��һ�ֳ������ʿ������Ե缫���ϣ�������������Ϊ�����������壬��B��CԪ�أ�����CO2��A��Eͬ���壬��Eԭ����������B������E��Na��B��C��Dͬ���ڣ����ڵڶ����ڣ�D��G������������ȣ�����ͬ���壬G��������ΪD��2������DΪOԪ�ء�GΪSԪ�أ�Hԭ������������HΪCl��Cԭ����������̼����֮�䣬��CΪNԪ�أ�B��D��G��������֮�͵���F��H��������֮�ͣ���F������Ϊ6+8+16-17=13����FΪAl��I�������ճ��������������Ľ������ױ���ʴ������IΪFe��
��1��IΪFeԪ�أ����ڵ������ڵڢ��壬�ʴ�Ϊ���������ڵڢ��壻
��2������CO2��������̼ԭ������ԭ��֮���γ�2�Թ��õ��Ӷԣ��ṹʽΪO=C=O���ʴ�Ϊ��O=C=O��
��3��A����Ԫ�ء�̼Ԫ�ؿ����γ�������A��ȷ��
B��DΪOԪ�أ�GΪSԪ�أ��ǽ�����O��S�������ȶ��ԣ�H2O��H2S����B����
C��HΪ��Ԫ�أ��ǽ�����Cl��S����Ԫ��G������������Ӧˮ��������Ա�H��������C��ȷ��
D�����Ӳ�ṹ��ͬ���˵����Խ�����Ӱ뾶ԽС�����Ӳ�Խ�����Ӱ뾶Խ�����Ӱ뾶�Ĵ�С˳��r��O2-����r��Na+����r��Al3+������D����
E��ˮ����֮����������������ΪҺ̬��������Ϊ���壬�е㣺H2O��H2S����E����
F��ͬ��ͬѹ�£���a L NH3��b L HClͨ��ˮ�У���������Һ��pH=7�����ݵ���غ���Һ��c��NH4+��=c��Cl-���������غ��֪��c��NH4+��+c��c��NH3��H2O����c��Cl-������ͬ��ͬѹ�£�NH3��������ڣ���a��b����F��ȷ��
�ʴ�Ϊ��BDE��
��4��Al��OH��3��ȫ������pH��4.7��Fe��OH��3��ȫ������pH��2.8���������ӿ�ʼ����ʱPHԽС��˵�������������ܽ��ԽС��������ɽṹ��ͬ����Fe��OH��3���ܶȻ���С��Al��OH��3�ܶȻ��ϴʴ�Ϊ��Al��OH��3��
��5������Cl��Fe��ɵ�ij�ֻ��������Һ���У�����ͭƬ����Һ��������Ϊ��ɫ������ΪFeCl3������������ԭ��Ӧ�������ӻ�õ��������������ӣ������缫��ӦʽΪ��Fe3++e-=Fe2+���ʴ�Ϊ��Fe3++e-=Fe2+��
��6������ʯī�缫��⺬��0.04mol CuSO4��0.04mol NaCl�Ļ����Һ400mL����������������Ӧ����������ȫ�ŵ�õ�����Ϊ0.02mol��ʵ����������Ϊ
=0.035mol������������Ϊ0.035mol-0.02mol=0.015mol��ת�Ƶ���Ϊ0.04mol+0.015mol��4=0.1mol����Һ��ͭ������ȫ�ŵ��õ���Ϊ0.08mol��С��0.1mol���ɵ���غ��жϵ�����ҺΪH2SO4��Na2SO4�����ݵ���غ�n��H+��+0.04mol=0.04mol��2����n��H+��=0.04mol����c��H+��=
=0.1mol/L��pH=-lgc��H+��=1���ʴ�Ϊ��1��
��1��IΪFeԪ�أ����ڵ������ڵڢ��壬�ʴ�Ϊ���������ڵڢ��壻
��2������CO2��������̼ԭ������ԭ��֮���γ�2�Թ��õ��Ӷԣ��ṹʽΪO=C=O���ʴ�Ϊ��O=C=O��
��3��A����Ԫ�ء�̼Ԫ�ؿ����γ�������A��ȷ��
B��DΪOԪ�أ�GΪSԪ�أ��ǽ�����O��S�������ȶ��ԣ�H2O��H2S����B����
C��HΪ��Ԫ�أ��ǽ�����Cl��S����Ԫ��G������������Ӧˮ��������Ա�H��������C��ȷ��
D�����Ӳ�ṹ��ͬ���˵����Խ�����Ӱ뾶ԽС�����Ӳ�Խ�����Ӱ뾶Խ�����Ӱ뾶�Ĵ�С˳��r��O2-����r��Na+����r��Al3+������D����
E��ˮ����֮����������������ΪҺ̬��������Ϊ���壬�е㣺H2O��H2S����E����
F��ͬ��ͬѹ�£���a L NH3��b L HClͨ��ˮ�У���������Һ��pH=7�����ݵ���غ���Һ��c��NH4+��=c��Cl-���������غ��֪��c��NH4+��+c��c��NH3��H2O����c��Cl-������ͬ��ͬѹ�£�NH3��������ڣ���a��b����F��ȷ��
�ʴ�Ϊ��BDE��
��4��Al��OH��3��ȫ������pH��4.7��Fe��OH��3��ȫ������pH��2.8���������ӿ�ʼ����ʱPHԽС��˵�������������ܽ��ԽС��������ɽṹ��ͬ����Fe��OH��3���ܶȻ���С��Al��OH��3�ܶȻ��ϴʴ�Ϊ��Al��OH��3��
��5������Cl��Fe��ɵ�ij�ֻ��������Һ���У�����ͭƬ����Һ��������Ϊ��ɫ������ΪFeCl3������������ԭ��Ӧ�������ӻ�õ��������������ӣ������缫��ӦʽΪ��Fe3++e-=Fe2+���ʴ�Ϊ��Fe3++e-=Fe2+��
��6������ʯī�缫��⺬��0.04mol CuSO4��0.04mol NaCl�Ļ����Һ400mL����������������Ӧ����������ȫ�ŵ�õ�����Ϊ0.02mol��ʵ����������Ϊ
| 0.784L |
| 22.4L/mol |
| 0.04mol |
| 0.4L |
����������ṹ����λ�ù�ϵ���ܶȻ���ԭ��ء�������ȣ��ƶ�Ԫ���ǽ���ؼ�����Ŀ�ۺϽϴ���Ҫѧ���߱���ʵ�Ļ�������4���м���Ϊ�״��㡢�ѵ㣬�漰��Ӧ���̸��ӣ����ù��̷��dz���������Ŀ�����غ㷨�ж�������Һ���ʡ�����������Ũ�ȣ�ʹ���㻯��Ϊ���ѶȽϴ�

��ϰ��ϵ�д�
 ��������ܸ�ϰϵ�д�
��������ܸ�ϰϵ�д�
�����Ŀ
�������ӷ���ʽ��ȷ���ǣ�������
| A����NaHSO4��Һ�еμ�Ba��OH��2��SO42-������ȫ��2H++SO42-+Ba2++2OH-=BaSO4��+2H2O |
| B�������ʯ��ˮ�м��������NaHCO3��Һ��Ca2++OH-+HCO3-�TCaCO3��+H2O |
| C��FeBr2��Һ�м����������ˮ 2Fe2++2Br-+2Cl2�TBr2+2Fe3++4Cl- |
| D����NaAlO2��Һ��ͨ������CO2��2AlO2-+CO2+3H2O=2Al��OH��3��+CO32- |
���й��ڱ���ϩ���ṹ��ʽΪ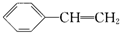 ����������������ǣ�������
����������������ǣ�������
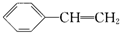 ����������������ǣ�������
����������������ǣ�������| A�����Ժ���ˮ��Ӧ |
| B������ϩ��ͬϵ�� |
| C����ѧʽΪC8H8 |
| D��1 mol����ϩ�����Ժ�4 mol H2�����ӳɷ�Ӧ |
���и���������ָ������Һ���ܴ���������ǣ�������
| A����pH=1����Һ�У�Fe2+��NO3-��SO42-��Na+ |
| B�����ܽ�Al��OH��3����Һ�У�NH4+��SO42-��Cl-��HCO3- |
| C����̪�ʺ�ɫ����Һ�У�K+��Na+��AlO2-��NO3- |
| D�����д���Fe2+����Һ�У�H+��K+��ClO-��SO42- |
 ����ÿ�տ�ѧ��վ���±���������÷���������о���Ա�����̲躬������������Ѫ��ϸ���ijɷ�-����ûʳ�Ӷ�����ûʳ����������EGCG�����о���ʾ���óɷ�ͨ������Ѫ��ϸ������������źŴ��ݣ���ʹѪ������Ѫ�����а�ϸ����ɱ����������֪EGCG�Ľṹ��ʽ��ͼ���й�EGCG��˵������ȷ���ǣ�������
����ÿ�տ�ѧ��վ���±���������÷���������о���Ա�����̲躬������������Ѫ��ϸ���ijɷ�-����ûʳ�Ӷ�����ûʳ����������EGCG�����о���ʾ���óɷ�ͨ������Ѫ��ϸ������������źŴ��ݣ���ʹѪ������Ѫ�����а�ϸ����ɱ����������֪EGCG�Ľṹ��ʽ��ͼ���й�EGCG��˵������ȷ���ǣ�������| A��EGCG�����к�����������̼ԭ�� |
| B��EGCG��FeCl3��Һ�ܷ�����ɫ��Ӧ |
| C��EGCG�ڿ����в��ױ����� |
| D��EGCG��������������Һ��Ӧ |


