��Ŀ����
8��A��B��C��D��Ϊ��ѧ��ѧ�ij��������Ҿ�����ͬһ��Ԫ�أ�����֮���ת����ϵ����ͼ��ʾ����Ӧ���������������Ѿ���ȥ����A$\stackrel{O_{2}}{��}$B$\stackrel{O_{2}}{��}$C$\stackrel{H_{2}O}{��}$D��1����AΪH2S��D��ˮ��Һ��ʹʪ�����ɫʯ����ֽ��죬BΪ�̼�����ζ���壬д��B��Cת���Ļ�ѧ��Ӧ����ʽ��2SO2+O2$\frac{\underline{����}}{��}$2SO3��
��2����A��ˮ��Һ��ʹʪ��ĺ�ɫʯ����ֽ������D��ϡ��Һ��ʹʪ�����ɫʯ����ֽ��죺A�Ļ�ѧʽΪNH3��ʵ�����Ʊ�����A�Ļ�ѧ��Ӧ����ʽ2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��A��Bת���Ļ�ѧ����ʽΪ4NH3+5O2 $\frac{\underline{����}}{��}$4NO+6H2O��
��3����AΪ���ý���Ԫ�صĵ��ʣ�DΪǿ���ɫ��Ӧ�Ի�ɫ����B�ǣ��ѧʽ��Na2O��C����Ϊ������ߵ�������C�������̼������Ӧ�Ļ�ѧ����ʽΪ2Na2O2+2CO2=2Na2CO3+O2��C��ˮ������Ӧ�����ӷ���ʽΪ2Na2O2+2H2O=4Na++4OH-+O2����
���� ��1����AΪH2S��D��ˮ��Һ��ʹʪ�����ɫʯ����ֽ��죬BΪ�̼�����ζ���壬��BΪSO2��CΪSO3��DΪH2SO4��
��2����A��ˮ��Һ��ʹʪ��ĺ�ɫʯ����ֽ������D��ϡ��Һ��ʹ��ɫ��ʪ��ʯ����ֽ��죬��AΪNH3��BΪNO��CΪNO2��DΪHNO3��
��3����AΪ���ý���Ԫ�صĵ��ʣ�DΪǿ���ɫ��Ӧ�Ի�ɫ����AΪNa��BΪNa2O��CΪNa2O2��DΪNaOH���ݴ˴��⣮
��� �⣺��1����AΪH2S��D��ˮ��Һ��ʹʪ�����ɫʯ����ֽ��죬BΪ�̼�����ζ���壬��BΪSO2��CΪSO3��DΪH2SO4����B��C�Ļ�ѧ����ʽ�ֱ�Ϊ2SO2+O2$\frac{\underline{����}}{��}$2SO3���ʴ�Ϊ��2SO2+O2$\frac{\underline{����}}{��}$2SO3��
��2����A��ˮ��Һ��ʹʪ��ĺ�ɫʯ����ֽ������D��ϡ��Һ��ʹ��ɫ��ʪ��ʯ����ֽ��죬��AΪNH3��BΪNO��CΪNO2��DΪHNO3��A��Bת���Ļ�ѧ����ʽ��4NH3+5O2 $\frac{\underline{����}}{��}$4NO+6H2O��ʵ�����Ʊ�NH3�Ļ�ѧ��Ӧ����ʽ Ϊ2NH4Cl+Ca��OH��2 $\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
�ʴ�Ϊ��NH3�� 2NH4Cl+Ca��OH��2 $\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��4NH3+5O2 $\frac{\underline{����}}{��}$4NO+6H2O��
��3����AΪ���ý���Ԫ�صĵ��ʣ�DΪǿ���ɫ��Ӧ�Ի�ɫ����AΪNa��BΪNa2O��CΪNa2O2��DΪNaOH��C�������̼������Ӧ�Ļ�ѧ����ʽΪ2Na2O2+2CO2=2Na2CO3+O2��C��ˮ������Ӧ�����ӷ���ʽΪ2Na2O2+2H2O=4Na++4OH-+O2�����ʴ�Ϊ��Na2O��2Na2O2+2CO2=2Na2CO3+O2��2Na2O2+2H2O=4Na++4OH-+O2����
���� ���⿼��Ԫ�ػ�������ƶϣ��漰S��N��NaԪ�ص��ʼ��仯����������ת������Ҫѧ����������Ԫ�ػ�����֪ʶ��ע����ػ���֪ʶ�Ļ��ۣ��ѶȲ���

 ��У����ϵ�д�
��У����ϵ�д�| ѡ�� | �������ʵ | �� �� |
| A | �ú������۵���С����ʳƷһ���ܷ��װ | �������۵���ˮ�� |
| B | �������ʴ�̲��� | SiO2���������������������� |
| C | ������������������еĹ����� | ����������ǿ��������������������̼ |
| D | �����ź�������ɽ��ɽ��������翼䡱�����˺�����¥���� | ��һ����Ȼ�����뽺��֪ʶ�й� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | �ð�ˮ��ȥ�Թ��ڱ��ϵ����� | |
| B�� | ��ͨ�������ķ�����ȥ�����е���ϩ | |
| C�� | �õ�ȼ�ķ��������Ҵ����������Ȼ�̼ | |
| D�� | ����ˮ���𱽡��ױ����ұ� |
| ��� | ʵ�� | ���� |
| A | ����ˮ�ķ�Ӧ | �����Ӵ�ú����ȡ�������ƣ������̶���С������װ��ˮ���ձ��� |
| B | ����һ��Ũ�ȵ��Ȼ�����Һ500mL | ȷ��ȡ�Ȼ��ع��壬���뵽500mL������ƿ�У���ˮ�ܽ⡢��ҡ�ȡ����� |
| C | �������ķ��� | �����������ƿ���ȶ���ʹ����������Ʈ���ǿ� |
| D | ȡ����Һ©����������ϲ�Һ�� | �²�Һ��ӷ�Һ©���¶˹ܿڷų����رջ�������һ���ձ����ϲ�Һ������ӷ�Һ©���¶˹ܿڷų� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ���εĹ������ڻ�ѧ�仯 | B�� | ��֭����Ҫ�ɷ�Ϊ���� | ||
| C�� | ���ö�֭��������ĭ���� | D�� | ���붹֭���Խ���NaCl�ܽ�� |
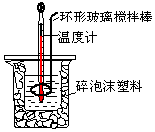 ��50mL0.50mol/L������50mL 0.55mol/L NaOH��Һ������ͼ��ʾ��װ���н����кͷ�Ӧ���ⶨǿ����ǿ�Ӧ�ķ�Ӧ�ȣ�
��50mL0.50mol/L������50mL 0.55mol/L NaOH��Һ������ͼ��ʾ��װ���н����кͷ�Ӧ���ⶨǿ����ǿ�Ӧ�ķ�Ӧ�ȣ�| ��ʼ�¶�t1/�� | ��ֹ�¶� t2/�� | �¶Ȳ� ��t2-t1��/�� | |||
| HCl | NaOH | ƽ��ֵ | |||
| 1 | 25.5 | 25.0 | 25.25 | 28.5 | 3.25 |
| 2 | 24.5 | 24.5 | 24.50 | 27.5 | 3.00 |
| 3 | 25.0 | 24.5 | 24.75 | 26.5 | 1.75 |
��2��װ���д�С�ձ�֮����������ĭ���ϵ�Ŀ���Ǽ���ʵ������е�������ʧ��
��3��ijͬѧʵ���¼�����������ʾ�����м�¼����ֹ�¶���ָ��ȫ��Ӧ������Һ������¶ȣ�
��4�������ʵ�鷢���кͷ�Ӧʱ�ų�������Ϊ1.31kJ���кͺ����ɵ���Һ�ı�����c=4.18J/��g•�棩��
��5�������50mL 0.55mol/L�İ�ˮ��NH3•H2O������NaOH��Һ��������ʵ�飬ͨ����õķ�Ӧ�ȼ����к��ȣ����к��ȡ�H��ƫ����ԭ����һˮ�ϰ�Ϊ������ʣ���Ӧ�����е�����Ҫ�����������ʷų�������ƫ�٣�
 ��ͼ��ʾ��װ�ã���������ƿ��װ�б����ᡢ�Ҵ���Ũ����ͷ�ʯ��ˮԡ���ȣ�����������������Ӧһ��ʱ����ֲ�Ʒ����װ��ˮ���ձ��У�Һ���Ϊ���㣬�������ַ�����������������ˮ�㣺
��ͼ��ʾ��װ�ã���������ƿ��װ�б����ᡢ�Ҵ���Ũ����ͷ�ʯ��ˮԡ���ȣ�����������������Ӧһ��ʱ����ֲ�Ʒ����װ��ˮ���ձ��У�Һ���Ϊ���㣬�������ַ�����������������ˮ�㣺 2016��9��4�գ�G20����ٿ���������ճ��ֳ��ɾ�����ɫ���Ʋ��������ɢ����գ��������������˳����ġ�ˮ���족��
2016��9��4�գ�G20����ٿ���������ճ��ֳ��ɾ�����ɫ���Ʋ��������ɢ����գ��������������˳����ġ�ˮ���족��