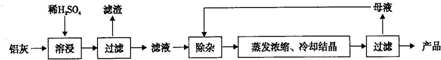
��Ŀ����
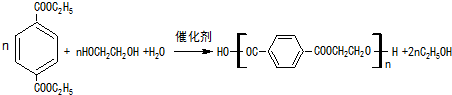
5������̪���ҹ���ʷ�ϵ��������������Ĺ���һ����ҩ��Ҳ�ǹ������������ڼ���ȱѪ�������ж���������ڵĴ���ҩ�����ҿƼ��������Ƚ����ϳɶ���̪��һ��·�����£�
��֪����

��E�ĺ˴Ź�������ֻ��һ��壻
��C�ܷ���������Ӧ��
�ش��������⣺
��1��D�Ļ�ѧ������2-����ϩ��C�к��еĹ���������ԭ�ӣ�
��2��A��B�ķ�Ӧ����Ϊȡ����Ӧ��B��C�ķ�Ӧ����Ϊ������Ӧ��
��3��H�Ľṹ��ʽΪ
 ��
����4����D����E�Ļ�ѧ����ʽΪ
 +HBr����CH3��3CBr��
+HBr����CH3��3CBr����5��K��C��Ϊͬϵ���Է���������C��14����˴Ź���������4��壬�ҷ����֮��Ϊ2��2��1��1����K�Ľṹ��ʽΪ
 ��
����6��������������̪�ĺϳ�·�ߣ����һ���Լ�ȩ�ͻ�����AΪԭ�Ϻϳ�2-�����Ҵ���
 ��
���ĺϳ�·�ߣ�
 ��
��
���� E�ĺ˴Ź�������ֻ��һ��壬��EΪ��CH3��3CBr����֪DΪ ��������Ϣ�ٿ�֪FΪ��CH3��3CMgBr���ɶ���̪�Ľṹ��֪HΪ
��������Ϣ�ٿ�֪FΪ��CH3��3CMgBr���ɶ���̪�Ľṹ��֪HΪ ����֪A��B��C�к��б�������AΪ
����֪A��B��C�к��б�������AΪ ��BΪ
��BΪ ��C�ܷ���������Ӧ����CΪ
��C�ܷ���������Ӧ����CΪ ��GΪ
��GΪ ��
��
��6���ɼ�ȩ�� �ϳ�2һ�����Ҵ��������üױ����������������·���ȡ������һ�ȼױ�������þ��������������
�ϳ�2һ�����Ҵ��������üױ����������������·���ȡ������һ�ȼױ�������þ�������������� ��
�� �����ȩ��Ӧ�ɵ�2һ�����Ҵ���
�����ȩ��Ӧ�ɵ�2һ�����Ҵ���
��� �⣺E�ĺ˴Ź�������ֻ��һ��壬��EΪ��CH3��3CBr����֪DΪ ��������Ϣ�ٿ�֪FΪ��CH3��3CMgBr���ɶ���̪�Ľṹ��֪HΪ
��������Ϣ�ٿ�֪FΪ��CH3��3CMgBr���ɶ���̪�Ľṹ��֪HΪ ����֪A��B��C�к��б�������AΪ
����֪A��B��C�к��б�������AΪ ��BΪ
��BΪ ��C�ܷ���������Ӧ����CΪ
��C�ܷ���������Ӧ����CΪ ��GΪ
��GΪ ��
��
��1��DΪ ����ѧ������2-����ϩ��CΪ
����ѧ������2-����ϩ��CΪ �����еĹ�������ȩ������ԭ�ӣ�
�����еĹ�������ȩ������ԭ�ӣ�
�ʴ�Ϊ��2-����ϩ��ȩ������ԭ�ӣ�
��2��A��B�ķ�Ӧ����Ϊȡ����Ӧ��B��C�ķ�Ӧ����Ϊ������Ӧ��
�ʴ�Ϊ��ȡ����Ӧ��������Ӧ��
��3��H�Ľṹ��ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��4����D����E�Ļ�ѧ����ʽΪ�� +HBr����CH3��3CBr��
+HBr����CH3��3CBr��
�ʴ�Ϊ�� +HBr����CH3��3CBr��
+HBr����CH3��3CBr��
��5��K��C�� ����Ϊͬϵ���Է���������C��14����˴Ź���������4��壬�ҷ����֮��Ϊ2��2��1��1����K�Ľṹ��ʽΪ
����Ϊͬϵ���Է���������C��14����˴Ź���������4��壬�ҷ����֮��Ϊ2��2��1��1����K�Ľṹ��ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��6���ɼ�ȩ�� �ϳ�2һ�����Ҵ��������üױ����������������·���ȡ������һ�ȼױ�������þ��������������
�ϳ�2һ�����Ҵ��������üױ����������������·���ȡ������һ�ȼױ�������þ�������������� ��
�� �����ȩ��Ӧ�ɵ�2һ�����Ҵ����ϳ�·��Ϊ
�����ȩ��Ӧ�ɵ�2һ�����Ҵ����ϳ�·��Ϊ ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���л�����ƶ���ϳɣ���������л�����ӡ�J�Ľṹ�뷴Ӧ�����ƶϣ�ע�����Ŀ��Ϣ�����⣬�������չ����ŵ�������ת������Ŀ�Ѷ��еȣ�

| ������ | �� | �� | �� | |
| A | Fe3O4 | CH3COOH | NH3•H2O | ��NH4��2SO 4 |
| B | SO2 | H2SO4 | NaOH | Na2O2 |
| C | CO | HNO3 | Cu2��OH��2CO3 | FeSO4 |
| D | H2O | NaHSO4 | Ba��OH��2 | Na2CO3 |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ������غ����������������ʵ�������Һ�з�Ӧ��Ba2++OH-+H++SO42-=BaSO4��+2H2O | |
| B�� | ̼������ڴ����У�CaCO3+2H+=Ca2++CO2��+2H2O | |
| C�� | ����������̼�����Լط�Ӧ�� CO2+2OH-=CO32-+H2O | |
| D�� | �Ƹ�ˮ��Ӧ��Na+2H2O=Na++2OH-+H2�� |
��һ�������£�MnO4-����Mn2+��Ӧ����MnO2��
��֪�������������������pH
| Al��OH��3 | Fe��OH��2 | Fe��OH��3 | |
| ��ʼ����ʱ | 3.4 | 6.3 | 2.7 |
| ��ȫ����ʱ | 5.2 | 9.7 | 3.2 |
��2��������Һ�л�����Fe2+�ķ�����ȡ������Һ���μ�KMnO4��Һ��KMnO4��Һ��ɫ��ע���Լ�������
��3�������ӡ����������¼������裺
������Һ�м������KMnO4��Һ��������Һ��pHԼΪ3.2��
���ȣ�����������ɫ���������ã��ϲ���Һ���Ϻ�ɫ��
����MnSO4���Ϻ�ɫ��ʧ�����ˣ�
�ٲ�����Ŀ���ǽ�Fe2+����ΪFe3+��������Һ��pHΪ3.2��Ŀ���ǵ���pHֵʹ��Ԫ�س�����ȫ��
�����ij����м���ŨHCl�����ȣ���˵�������д���MnO2���������л���ɫ�������ɣ�
�ۢ��м���MnSO4��Ŀ���dz�ȥ������MnO4-��
��4���Ӷ��ѭ��ʹ�ú�ĸҺ�пɻ��յ���Ҫ������K2SO4���ѧʽ����
| A�� | ��С�մ�����θ����� | |
| B�� | ��ʯ����ʳƷ����� | |
| C�� | ��ĥ��ʯ��ָ���� | |
| D�� | �����ظ���ؼ���˾���Ƿ�ƺ�ݳ� |
| A�� |  | B�� |  | C�� |  | D�� |  |
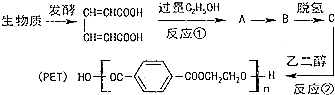
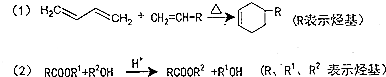
 ��
�� ��
��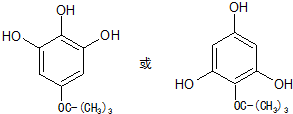 ����дһ�֣�
����дһ�֣� ���������л�������һ�ֺϳ�·�ߣ�
���������л�������һ�ֺϳ�·�ߣ�
 ��A����B�ķ�Ӧ�����Ǽӳɷ�Ӧ��
��A����B�ķ�Ӧ�����Ǽӳɷ�Ӧ�� ��
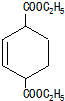
�� ��ͬ���칹���з���������������10�֣������������칹����
��ͬ���칹���з���������������10�֣������������칹���� ����ṹ��ʽ����
����ṹ��ʽ���� �ĺϳ�·�ߣ�
�ĺϳ�·�ߣ� $��_{Cu/��}^{O_{2}}$
$��_{Cu/��}^{O_{2}}$ $��_{һ������}^{HCN}$
$��_{һ������}^{HCN}$ $\stackrel{H_{2}O/H+}{��}$
$\stackrel{H_{2}O/H+}{��}$ $��_{��}^{ŨH_{2}SO_{4}}$
$��_{��}^{ŨH_{2}SO_{4}}$ ��
��
 ��
�� ��
��