��Ŀ����
���Ϊ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ�أ�����Ӧ�Ļ�ѧʽ��գ�
��1��Ԫ�آ�λ�����ڱ��� ����������Ų�ʽΪ ��
��2��Ԫ�آ���Ԫ�����ڱ��е�λ���� ����ԭ�ӽṹʾ��ͼΪ�� ��
��3��Ԫ�آݵ�ԭ�ӵļ۲�����Ų�ͼΪ ���������ӵĵ���ʽΪ ��
��4��Ԫ�آ�����γɵij����������������ԭ�ӵ��ӻ������� ��VSEPRģ���� �����ӿռ乹���� ��
��5��Ԫ�آݢޢߢ��γɵļ����Ӱ뾶�ɴ�С˳��Ϊ ��
��6���٢ۢܢ��е�ij����Ԫ�ؿ��γɾ���10�����ӵĻ�Ϊ�ȵ���������������仯ѧʽ�ֱ�Ϊ �� �� �� ��
��7��������������ӵ�ˮ��Һ����μӰ�ˮ����������Ӧ�漰�����ӷ���ʽΪ�� �� ���������������������λ��Ϊ ��
| �� | |||||||||||||||||
| �� | �� | �� | �� | �� | |||||||||||||
| �� | �� | ||||||||||||||||
| �� | �� |
��2��Ԫ�آ���Ԫ�����ڱ��е�λ����
��3��Ԫ�آݵ�ԭ�ӵļ۲�����Ų�ͼΪ
��4��Ԫ�آ�����γɵij����������������ԭ�ӵ��ӻ�������
��5��Ԫ�آݢޢߢ��γɵļ����Ӱ뾶�ɴ�С˳��Ϊ
��6���٢ۢܢ��е�ij����Ԫ�ؿ��γɾ���10�����ӵĻ�Ϊ�ȵ���������������仯ѧʽ�ֱ�Ϊ
��7��������������ӵ�ˮ��Һ����μӰ�ˮ����������Ӧ�漰�����ӷ���ʽΪ��
���㣺Ԫ�������ɺ�Ԫ�����ڱ����ۺ�Ӧ��
ר�⣺Ԫ����������Ԫ�����ڱ�ר��
��������Ԫ�������ڱ���λ��֪��ΪH����ΪBe����ΪC����ΪN����ΪO����ΪF����ΪMg����ΪCl����ΪFe����ΪCu��
��1��Ԫ�آ�λ�����ڱ���ds�������ݺ�������Ų�������д������Ų�ʽ��
��2����Ԫ�آ���Ԫ�����ڱ��е�λ�ã���֪��λ�ڵ������ڵڢ��壻ԭ�Ӻ�����26�����ӣ��ɺ�������Ų����ɣ���֪���������Ϊ2��8��14��2��
��3��Ԫ�آݵ�ԭ�ӵļ۲�����Ų�ʽΪ2s22p4���ݴ˻�����۵����Ų�ͼ��O2-�������8�����ӣ���2����λ����ɣ�
��4��Ԫ�آ�����γɵij���������ΪNH3������Nԭ�Ӽ۲���Ӷ�����ȷ���ӻ����ͣ�VSEPRģ�ͣ����Nԭ�ӹµ��Ӷ������۲���Ӷ���ȷ����ռ乹�ͣ�
��5�����Ӳ�ṹ��ͬ���˵����Խ�����Ӱ뾶ԽС�����Ӳ�Խ�����Ӱ뾶Խ��
��6��ԭ��������ͬ���۵����������������������ͬ������Ϊ�ȵ����壬���10���������
��7������ˮ���뵽����ͭ���ӵ���Һ�У��Ȳ���������ͭ��ɫ������Ȼ��������ܽⲢ�õ��İ���ͭ�����ӣ�
��1��Ԫ�آ�λ�����ڱ���ds�������ݺ�������Ų�������д������Ų�ʽ��
��2����Ԫ�آ���Ԫ�����ڱ��е�λ�ã���֪��λ�ڵ������ڵڢ��壻ԭ�Ӻ�����26�����ӣ��ɺ�������Ų����ɣ���֪���������Ϊ2��8��14��2��
��3��Ԫ�آݵ�ԭ�ӵļ۲�����Ų�ʽΪ2s22p4���ݴ˻�����۵����Ų�ͼ��O2-�������8�����ӣ���2����λ����ɣ�
��4��Ԫ�آ�����γɵij���������ΪNH3������Nԭ�Ӽ۲���Ӷ�����ȷ���ӻ����ͣ�VSEPRģ�ͣ����Nԭ�ӹµ��Ӷ������۲���Ӷ���ȷ����ռ乹�ͣ�
��5�����Ӳ�ṹ��ͬ���˵����Խ�����Ӱ뾶ԽС�����Ӳ�Խ�����Ӱ뾶Խ��
��6��ԭ��������ͬ���۵����������������������ͬ������Ϊ�ȵ����壬���10���������
��7������ˮ���뵽����ͭ���ӵ���Һ�У��Ȳ���������ͭ��ɫ������Ȼ��������ܽⲢ�õ��İ���ͭ�����ӣ�
���
�⣺��Ԫ�������ڱ���λ��֪��ΪH����ΪBe����ΪC����ΪN����ΪO����ΪF����ΪMg����ΪCl����ΪFe����ΪCu��
��1��Ԫ�آ�λ�����ڱ���ds�������ݺ�������Ų����ɣ�������Ų�ʽΪ��1s22s22p63s23p63d104s1��
�ʴ�Ϊ��ds��1s22s22p63s23p63d104s1��
��2����Ԫ�آ���Ԫ�����ڱ��е�λ�ã���֪��λ�ڵ������ڵڢ��壻ԭ�Ӻ�����26�����ӣ��ɺ�������Ų����ɣ���֪���������Ϊ2��8��14��2��ԭ�ӽṹʾ��ͼΪ�� ��
��
�ʴ�Ϊ���������ڵڢ��壻 ��
��
��3��Ԫ�آݵ�ԭ�ӵļ۲�����Ų�ʽΪ2s22p4����۵����Ų�ͼΪ ��O2-�������8�����ӣ���2����λ����ɣ�����ʽΪ��
��O2-�������8�����ӣ���2����λ����ɣ�����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
�� ��
��
��4��Ԫ�آ�����γɵij���������ΪNH3��Nԭ�Ӽ۲���Ӷ���=3+
=4����ȡsp3�ӻ���VSEPRģ��Ϊ�������壬Nԭ�ӹµ��Ӷ���Ϊ1����ռ乹��Ϊ�����Σ�
�ʴ�Ϊ��sp3���������壻�����Σ�
��5�����Ӳ�ṹ��ͬ���˵����Խ�����Ӱ뾶ԽС�����Ӳ�Խ�����Ӱ뾶Խ�����Ӱ뾶��r��Cl-����r��O2-����r��F-����r��Mg2+����
�ʴ�Ϊ��r��Cl-����r��O2-����r��F-����r��Mg2+����
��6���٢ۢܢ��е�ij����Ԫ�ؿ��γɾ���10�����ӵĻ�Ϊ�ȵ��������������ΪNH4+ ��CH4��H3O+�� NH3��
�ʴ�Ϊ��NH4+ ��CH4��H3O+�� NH3��
��7������ˮ���뵽����ͭ��Һ�У��Ȳ���������ͭ��ɫ������Ȼ��������ܽⲢ�õ�[Cu��NH3��4]2+�����ӣ�����������λ��Ϊ4����Ӧ�漰�����ӷ���ʽΪ��Cu2++2NH3��H2O�TCu��OH��2��+2NH4+��Cu��OH��2+4NH3�T[Cu��NH3��4]2++2OH-��
�ʴ�Ϊ��Cu2++2NH3��H2O�TCu��OH��2��+2NH4+��Cu��OH��2+4NH3�T[Cu��NH3��4]2++2OH-��4��
��1��Ԫ�آ�λ�����ڱ���ds�������ݺ�������Ų����ɣ�������Ų�ʽΪ��1s22s22p63s23p63d104s1��
�ʴ�Ϊ��ds��1s22s22p63s23p63d104s1��
��2����Ԫ�آ���Ԫ�����ڱ��е�λ�ã���֪��λ�ڵ������ڵڢ��壻ԭ�Ӻ�����26�����ӣ��ɺ�������Ų����ɣ���֪���������Ϊ2��8��14��2��ԭ�ӽṹʾ��ͼΪ��
 ��
���ʴ�Ϊ���������ڵڢ��壻
 ��
����3��Ԫ�آݵ�ԭ�ӵļ۲�����Ų�ʽΪ2s22p4����۵����Ų�ͼΪ
 ��O2-�������8�����ӣ���2����λ����ɣ�����ʽΪ��
��O2-�������8�����ӣ���2����λ����ɣ�����ʽΪ�� ��
���ʴ�Ϊ��
 ��
�� ��
����4��Ԫ�آ�����γɵij���������ΪNH3��Nԭ�Ӽ۲���Ӷ���=3+
| 5-1��3 |
| 2 |
�ʴ�Ϊ��sp3���������壻�����Σ�
��5�����Ӳ�ṹ��ͬ���˵����Խ�����Ӱ뾶ԽС�����Ӳ�Խ�����Ӱ뾶Խ�����Ӱ뾶��r��Cl-����r��O2-����r��F-����r��Mg2+����
�ʴ�Ϊ��r��Cl-����r��O2-����r��F-����r��Mg2+����
��6���٢ۢܢ��е�ij����Ԫ�ؿ��γɾ���10�����ӵĻ�Ϊ�ȵ��������������ΪNH4+ ��CH4��H3O+�� NH3��
�ʴ�Ϊ��NH4+ ��CH4��H3O+�� NH3��
��7������ˮ���뵽����ͭ��Һ�У��Ȳ���������ͭ��ɫ������Ȼ��������ܽⲢ�õ�[Cu��NH3��4]2+�����ӣ�����������λ��Ϊ4����Ӧ�漰�����ӷ���ʽΪ��Cu2++2NH3��H2O�TCu��OH��2��+2NH4+��Cu��OH��2+4NH3�T[Cu��NH3��4]2++2OH-��
�ʴ�Ϊ��Cu2++2NH3��H2O�TCu��OH��2��+2NH4+��Cu��OH��2+4NH3�T[Cu��NH3��4]2++2OH-��4��
�����������Ƕ����ʽṹ�Ŀ��飬�漰��������Ų����ӻ�������۲���ӶԻ������ۡ����ӽṹ�����뾶�Ƚϡ��ȵ����塢�����ȣ�ע�س���֪ʶ��Ŀ��飬��Ҫѧ���߱���ʵ�Ļ������Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
��״���£�ij��̬�л����ܶ�Ϊ3.125g/L������л�������ʽ�����ǣ�������
| A��CH |
| B��CH2 |
| C��CH3 |
| D��CH2O |
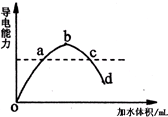 ��һ���¶��£��������ˮϡ�����У���Һ�ĵ���������ͼ��ʾ����ش�
��һ���¶��£��������ˮϡ�����У���Һ�ĵ���������ͼ��ʾ����ش�

 ��ͨ��״���£�AΪ��̬���ʣ�����ͼת����ϵ���ش�
��ͨ��״���£�AΪ��̬���ʣ�����ͼת����ϵ���ش�