��Ŀ����
����Ŀ����0.8 mol I2(g)��1.2 mol H2(g)����ij1L�ܱ������У���һ���¶��·�����Ӧ��I2(g)��H2(g) ![]() 2HI(g)���ﵽƽ�⡣HI�����������ʱ��ı仯�������ʾ��
2HI(g)���ﵽƽ�⡣HI�����������ʱ��ı仯�������ʾ��
HI������� | 1min | 2min | 3min | 4min | 5min | 6min | 7min[ |
����I | 26% | 42% | 52% | 57% | 60% | 60% | 60% |
����II | 20% | 33% | 43% | 52% | 57% | 65% | 65% |
��1��������I����ƽ��ʱ������÷�Ӧ��ƽ�ⳣ��K��Ҫ���г�������̡�
��2��������I�ӿ�ʼ��Ӧ������ƽ��ʱ��H2�ķ�Ӧ����Ϊ____________��
��3��Ϊ�ﵽ����II�����ݣ����ڷ�Ӧ��ϵ���ܸı�IJ�����_______________��
��4���÷�Ӧ����H__________0����">"��"<"��"="��
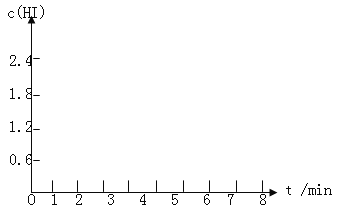
��5��������I�´ﵽƽ�����7minʱ���������ѹ��Ϊԭ����һ�롣����ͼ�л���c(HI)��ʱ��仯�����ߡ�
���𰸡���1����I2����Ũ��Ϊx
I2(g) + H2(g) ![]() 2HI(g)
2HI(g)
��ʼŨ�ȣ�mol/L����0.8 1.2 0
ת��Ũ�ȣ�mol/L����x x 2x
ƽ��Ũ�ȣ�mol/L����0.8-x 1.2-x 2x
HI���������Ϊ60%����2x/2=60%��x=0.6 mol/L
K=c2 (HI) /[c(H2)��c(I2)]=1.22/(0.2��0.6)=12��
��2��0.12 mol/(L��min)��3�������¶�����4��<��
��5��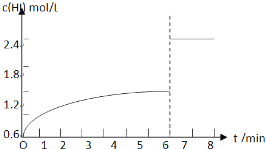
��������
�����������1���ɱ������ݿ�֪������I��5minʱ����ƽ��״̬����I2����Ũ��Ϊxmol/L����
I2��g��+H2��g��![]() 2HI��g��
2HI��g��
��ʼŨ����mol/L����0.8 1.2 0
ת��Ũ����mol/L����x x 2x
ƽ��Ũ����mol/L����0.8-x 1.2-x 2x
HI���������Ϊ60%������![]() =60%����x=0.6��ƽ�ⳣ��K= c2 (HI) /[c(H2)��c(I2)]=1.22/(0.2��0.6)=12���ʴ�Ϊ��12��
=60%����x=0.6��ƽ�ⳣ��K= c2 (HI) /[c(H2)��c(I2)]=1.22/(0.2��0.6)=12���ʴ�Ϊ��12��
��2��������I�ӿ�ʼ��Ӧ������ƽ��ʱ��H2�ķ�Ӧ����Ϊ![]() =0.12 mol/��Lmin�����ʴ�Ϊ��0.12 mol/��Lmin����
=0.12 mol/��Lmin�����ʴ�Ϊ��0.12 mol/��Lmin����
��3����ͬʱ����HI�����������С��˵����Ӧ���ʼ�����ƽ��ʱHI���������������Iʱ���ʸı�����ƽ�������ƶ�������ѹǿ��������Ӱ��ƽ���ƶ��������ǽ����¶ȣ��ʴ�Ϊ�������¶ȣ�
��4�������¶�ƽ��ʱ�����ƶ���˵������ӦΪ���ȷ�Ӧ������H��0���ʴ�Ϊ������
��5��������I�´ﵽƽ���HI��Ũ��Ϊ1.2mol/L����7minʱ���������ѹ��Ϊԭ����һ�룬ѹǿ����ƽ�ⲻ�ƶ���HI��Ũ�ȱ�Ϊԭƽ���2������HIŨ�ȱ�Ϊ2.4mol/L��c��HI����ʱ��仯������Ϊ��