��Ŀ����
5���������ƣ�NaClO2����һ��ǿ������Ư�����㷺���ڷ�֯��ӡȾ��ʳƷ��ҵ�����ڼ��Ի������ȶ����ڣ�ijͬѧ�������Ϻ��������NaClO2����Ҫ�������£�
��1��˫��ˮ�ĵ���ʽΪ
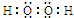 �����з�����Ӧ�Ļ�ԭ����Na2SO3���ѧʽ����
�����з�����Ӧ�Ļ�ԭ����Na2SO3���ѧʽ������2�����з�Ӧ�����ӷ���ʽ��2ClO2+H2O2+2OH-=2ClO2-+O2��+2H2O��
��3��ClO2��һ�ָ�Чˮ�������������������ƺ�ϡ����Ϊԭ���Ʊ���д���÷�Ӧ�Ļ�ѧ����ʽ��5NaClO2+4HCl=5NaCl+4ClO2��+2H2O��
��4��NaClO2���ʿɷֽ�ΪNaClO3��NaCl��ȡ����������ǰ���NaClO2�����������Һ���ֱ�������FeSO4��Һ��Ӧʱ������Fe2+�����ʵ�����ͬ�����ͬ����������ͬ�������жϡ���
���� ������ͼ��֪��Ӧ���з�Ӧ��ΪNaClO3��Na2SO3������A��Һ������C1O2��Na2SO4��Һ����Ӧ���ӷ���ʽΪ2H++SO32-+2ClO3-=2C1O2+SO42-+H2O������Na2SO3���л�ԭ���ǻ�ԭ������ClO3-��ԭΪC1O2����������NaClO2������һ����ClO2��NaClO2�����ϼ۽��ͣ�����ԭ����H2O2�ض�����������������������Ӧ��ClO2����������������ԭ��Ӧ��H2O2�ǻ�ԭ��������������Ӧ�����ݵ���ת���غ��֪4n��ClO2��=n��HCl�������Է�Ӧ����ʽΪ2NaOH+2ClO2+H2O2=2 NaClO2+2H2O+O2�����������Ӹ�Ĥ���أ��ӷ�Ӧ��֪�õ���������ɡ��Ӣ�֪�м����ɣ��������������ɣ����Ԣ�Ϊ��������Ʊ����ǵ��ˮ������2H++2e-=H2��������4OH--4e-=O2��+2H2O������AΪ���ᣮ
��1��˫��ˮ�ĽṹʽΪH-O-O-H��I��SԪ�صĻ��ϼ���+4������Ϊ+6�ۣ�
��2�������2NaOH+2ClO2+H2O2=2 NaClO2+2H2O+O2����
��3�����������ƺ�ϡ���ᷴӦ����ClO2��Ϊ������ԭ��Ӧ��
��4��NaClO2���ʿɷֽ�ΪNaClO3��NaCl������ʽΪ3NaClO2=2NaClO3+NaCl��NaClO2����������FeSO4��Һ��Ӧ3ClO2-+12Fe2++6H2O=4Fe��OH��3��+3Cl-+8Fe3+���Դ������
��� �⣺������ͼ��֪��Ӧ���з�Ӧ��ΪNaClO3��Na2SO3������A��Һ������C1O2��Na2SO4��Һ����Ӧ���ӷ���ʽΪ2H++SO32-+2ClO3-=2C1O2+SO42-+H2O������Na2SO3���л�ԭ���ǻ�ԭ������ClO3-��ԭΪC1O2����������NaClO2������һ����ClO2��NaClO2�����ϼ۽��ͣ�����ԭ����H2O2�ض�����������������������Ӧ��ClO2����������������ԭ��Ӧ��H2O2�ǻ�ԭ��������������Ӧ�����ݵ���ת���غ��֪4n��ClO2��=n��HCl�������Է�Ӧ����ʽΪ2NaOH+2ClO2+H2O2=2 NaClO2+2H2O+O2�����������Ӹ�Ĥ���أ��ӷ�Ӧ��֪�õ���������ɡ��Ӣ�֪�м����ɣ��������������ɣ����Ԣ�Ϊ��������Ʊ����ǵ��ˮ������2H++2e-=H2��������4OH--4e-=O2��+2H2O������AΪ���ᣮ
��1��˫��ˮ�ĽṹʽΪH-O-O-H������ʽΪ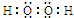 ��I��SԪ�صĻ��ϼ���+4������Ϊ+6�ۣ���ԭ��ΪNa2SO3��
��I��SԪ�صĻ��ϼ���+4������Ϊ+6�ۣ���ԭ��ΪNa2SO3��
�ʴ�Ϊ��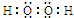 ��Na2SO3��
��Na2SO3��
��2�����з���2NaOH+2ClO2+H2O2=2 NaClO2+2H2O+O2�������ӷ�ӦΪ2ClO2+H2O2+2OH-=2ClO2-+O2��+2H2O��
�ʴ�Ϊ��2ClO2+H2O2+2OH-=2ClO2-+O2��+2H2O��
��3�����������ƺ�ϡ���ᷴӦ����ClO2��Ϊ������ԭ��Ӧ���ɵ��ӡ�ԭ���غ��֪��ӦΪ5NaClO2+4HCl=5NaCl+4ClO2��+2H2O��
�ʴ�Ϊ��5NaClO2+4HCl=5NaCl+4ClO2��+2H2O��
��4��NaClO2���ʿɷֽ�ΪNaClO3��NaCl��ClԪ�ش�+3�۱�Ϊ+5�ۺ�-1�ۣ���ӦΪ3NaClO2=2NaClO3+NaCl��NaClO2����������FeSO4��Һ��Ӧ3ClO2-+12Fe2++6H2O=4Fe��OH��3��+3Cl-+8Fe3+�����յõ�ClԪ����+3�۱�Ϊ-1�ۣ�����NaClO2����ǰ��ֱ���Fe2+��Ӧʱ�����վ��õ�����NaCl��ClԪ�ؾ���+3�۱�Ϊ-1�ۣ����ݵ����غ㣬���������еõ��ĵ��ӵ����ʵ�����ͬ������Fe2+�����ʵ�����ͬ��
�ʴ�Ϊ����ͬ��
���� ���⿼�����ʵ��Ʊ�ʵ�飬Ϊ��Ƶ���㣬�����Ʊ������з����ķ�Ӧ����ѧ��Ӧԭ��Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬ע�����ӷ�Ӧ��������ԭ��Ӧ��Ӧ�ã���Ŀ�ѶȲ���

| A�� | Cl-��Cu2+��SO42-��NO3- | B�� | Cl-��Na+��SO42-��CH3COO- | ||
| C�� | Cl-��Fe2+��MnO4-��NO3- | D�� | AlO2-��Na+��SO42-��NO3- |
| A�� | 1 mol��Լ���а���٤���������� | |
| B�� | 1 molCaCl2����2 mol Cl- | |
| C�� | 1 mol��������6.02��1023������ | |
| D�� | 1 molH2O����1 molH2��1 molO |
| A�� | �����Ӱ뾶��E��D | |
| B�� | ��̬�⻯����ȶ��ԣ�B��E | |
| C�� | ����������Ӧˮ����ļ��ԣ�C��D | |
| D�� | A��E�γɵĻ�����Ϊ���ӻ����� |
| A�� | 5mol/L | B�� | 3mol/L | C�� | 2mol/L | D�� | 7mol/L |
| A�� | ��ȥMgCl2��Һ��������FeCl3����ѡ��MgCO3 | |
| B�� | �����ʵ���Ũ�ȵģ�NH4��2SO4��Һ�ͣ�NH4��2CO3��Һ��NH4+��Ũ��ǰ�ߴ��ں��� | |
| C�� | NaHS��Һ�У���������CuCl2��Һ��������ɫ������HS-��ˮ��̶�����pH���� | |
| D�� | �ñ����Ȼ����Һ������ϴ����������⼣ |
| A�� | Fe3+��H+��Cl- SO42- | B�� | Na+��K+��Ba2+��HCO3- | ||
| C�� | Na+ Cl- NO3- K+ | D�� | K+ Na+ Cl- SO42- |
| A�� | �������ϩ���۵㶼��CH4�ĸ� | B�� | �������ϩ���ɷ����ӳɷ�Ӧ | ||
| C�� | �����ܷ���������Ӧ | D�� | ������ϩ���dz�������ԭ�� |
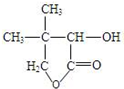 G�ĺϳ���·��ͼ��
G�ĺϳ���·��ͼ��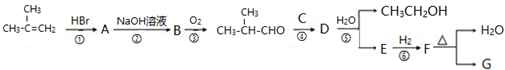
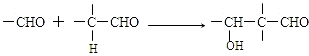
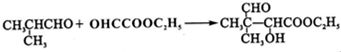 ��
��