��Ŀ����
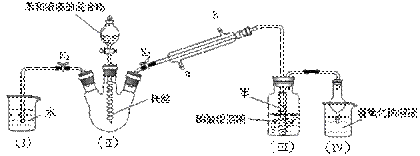
������ͼ��ʾװ�ý���ʵ�飬�о�NH3��ijЩ���ʣ�
��1���Ƚ���NH4��2Cr2O7�þƾ��Ƽ��ȣ��շֽ�ʱ����ƾ��ƣ���NH4��2Cr2O7�����ֽ�����N2��H2O��Cr2O3���壬���巢�졣��������ȱ䰵ʱ����Ũ��ˮ�й����������ֱ�������ҿ�ʼ����ƿ�ڵ��ܿڸ�����������������ɫ���̣����ָ�������йػ�ѧ����ʽΪ
______________________________��______________________________��
______________________________��______________________________��
��2���������ƿ�п�����������ɫ�����塣ʵ�����Cr2O3�������δ�䡣д����NH4��2Cr2O7�ֽ�Ļ�ѧ����ʽ________________________________________________________��
��3��ʵ��֤���˰��Ĵ�������_______________��Ӧ������ȡ������ȡ��������ݵ�ʵ��������________________________________________________________________��
��Ӧ�Ĵ�����_______________��
��1��4NH3+5O2![]() 4NO+6H2O
4NO+6H2O
2NO+O2====2NO2 3NO2+H2O====2HNO3+NO HNO3+NH3====NH4NO3
��2����NH4��2Cr2O7![]() N2��+4H2O+Cr2O3
N2��+4H2O+Cr2O3
��3������ ����ƾ��ƺ�����ʯ�����Ϲ�����ȱ䰵ʱ����Ũ��ˮ�йĿ�����ʹ����������������Ӧ�������ֱ����
Cr2O3
����������Ӧע��֪ʶ��Ǩ�ƣ�NH3��������������Ϥ��֪ʶ������ͻ�ƿ���������NH3�Ĵ���������ȷCr2O3�����ó�Ϊ��������ۡ�

 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д� ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д� ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д���2��ijѧ���ж�SO2��Na2O2��Ӧ�����������ƣ�����Ϊ�����жϺ����𣿣������������������__________����Ҫ˵������___________________________________________��
��3����ͬѧ��ȷ����Ӧ���Ƿ����������ɣ���������ͼ��ʾװ�ý���ʵ�顣
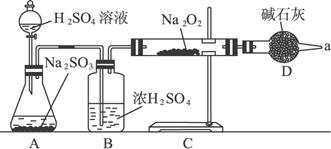
װ��B��������_________________________________________________��D�������ǣ�________________________________________��
��4��Ϊȷ�Ϸ�Ӧ���������±�������ʵ��
���� | ��������� |
��ȷ���Ƿ������������IJ����ǣ� |
|
��ȷ���Ƿ��������Ʋ����IJ����ǣ� |
|
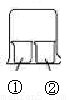
������ͼ��ʾװ�ý�������ʵ�飬ʵ������Ԥ�������һ�µ���
|
|
������� |
������� |
Ԥ��ٵ����� |
|
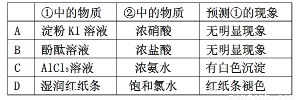
A |
����KI��Һ |
Ũ���� |
���������� |
|
B |
��̪��Һ |
Ũ���� |
���������� |
|
C |
AlCl3��Һ |
Ũ��ˮ |
�а�ɫ���� |
|
D |
ʪ���ֽ�� |
������ˮ |
��ֽ����ɫ |