��Ŀ����
14��ijʵ����Ҫ100mL��0.1mol/L��Na2CO3��Һ����ͨ�����²������ƣ��ٰѳ����õĹ���Na2CO3����С�ձ��У�����������ˮ�ܽ⣮Ϊ�ӿ��ܽ����ʹ�ò����������������ƣ�����
�ڰѢ�������Һ��ȴ�����º�С��ת��100��������ƿ�����������ƣ�
�ۼ���������ˮ��Һ�����̶���1��2cm�������ý�ͷ�ιܣ����������ƣ�С�ĵμ�����ˮ����Һ��Һ����͵���̶�������
������������ˮϴ�Ӳ��������ձ�2��3�Σ�ÿ��ϴ�ӵ���Һ��С��ת������ƿ��������ҡ��
�ݽ�����ƿ���������ҡ�ȣ�
��1������������ȷ��˳���Ǣ٢ڢܢۢݣ�����ţ���
��2����������Һ���ܶ�Ϊ1.06g/mL�������Һ����������Ϊ1%��
��3����ȡ��20mL���Na2CO3����Һ��������ˮϡ�ͳ�c��Na+��=0.01mol/L����Һ����ϡ�ͺ���Һ�����Ϊ400mL
��4��������100mL��0.1mol/L��Na2CO3��Һʱ�����в����е�ac�ᵼ�½��ƫ�ͣ����������д��
a�����ձ��е���Һת�Ƶ�����ƿʱ������������ƿ��
b������ʱ���ӿ̶���
c������ʱ���ӿ̶���
d���ɾ�������ƿδ�����������������Һ��
���� ����������һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢�������ܽ⡢��ȴ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿȷ����ȷ�IJ������裬ѡ����Ҫ��������
��Ҫ����100ml��ҺӦѡ��100ml������ƿ��
�۶���ʱʹ�ý�ͷ�ιܣ�
��1����������һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢�������ܽ⡢��ȴ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿȷ����ȷ�IJ������裻
��2������$c=\frac{1000��?}{M}$���㣻
��3��������Һϡ�Ͷ���CŨVŨ=CϡVϡ�����㣻
��4������c=n/V��������ʵ����ʵ���n����Һ�����V�ı仯��������������
��� �⣺���ܽ�ʱ��Ϊ�ӿ�̼�����ܽ���ٶȣ����ò��������裬�ʴ�Ϊ����������
��Ҫ����100ml��ҺӦѡ��100ml������ƿ���ʴ�Ϊ��100mL����ƿ��
�۶���ʱʹ�ý�ͷ�ι���μ��룬�ʴ�Ϊ����ͷ�ιܣ�
��1������һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢�������ܽ⡢��ȴ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������ȷ��˳��Ϊ���٢ڢܢۢݣ��ʴ�Ϊ���٢ڢܢۢݣ�
��2������$c=\frac{1000��?}{M}$��֪������Һ����������Ϊ?=$\frac{0.1��106}{1000��1.06}��100%=1%$���ʴ�Ϊ��1%��
��3��ϡ��ǰ�����ӵ�Ũ����0.2mol/L����ϡ�ͺ������ӵ�Ũ����0.01mol/L����ϡ�ͺ����Һ�����ΪVmL��������Һϡ�Ͷ���CŨVŨ=CϡVϡ�������֪��0.2mol/L��20mL=0.01mol/L��VmL�����V=400���ʴ�Ϊ��400��
��4������c=n/V��֪�����ձ��е���Һת�Ƶ�����ƿʱ������������ƿ�⣬�����ʼ��٣�Ũ��ƫС������ʱ���ӿ̶��ߣ�����Һ�������С������Ũ��ƫ�ߣ���֮����ʱ���ӿ̶��ߣ�Ũ��ƫ�ͣ��ɾ�������ƿδ�����������������Һ������Ӱ��ʵ�������ʴ�Ϊ��ac��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƹ����еļ���������������ڻ�������Ŀ���ѶȲ���

 ��У����ϵ�д�
��У����ϵ�д�| B12�ṹ��Ԫ | SF6 | S8 | HCN |
 |  |  |  |
| �۵�1873K | ���� | ������CS2 | ��Һ�������� |
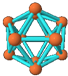
| A�� | ����B12�γɵľ������Ϊԭ�Ӿ��� | |
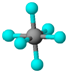
| B�� | SF6���ɼ��Լ����ɵķǼ��Է��� | |
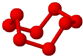
| C�� | S8��HCN�γɾ������������ͬ | |
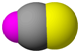
| D�� | HCN�γɾ�������е�������ֻ�й��ۼ� |
| A�� | �������Һ�м���ϡ���������ɫ���壬������ͨ�����ʯ��ˮ����Һ����ǣ�һ����CO32- | |
| B�� | �������Һ����μ�������������Һ����ʼ�а�ɫ�������ɣ������μ�����������Һ���������������ܽ⣬һ���� Al3+ | |
| C�� | �������Һ���ȵμ������ữδ���ֳ������ټ��� BaCl2 ��Һ�а�ɫ����������һ����SO42- | |
| D�� | �������Һ�еμ� NaOH ��Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬һ����NH4+ |
| A�� | 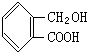 | B�� | 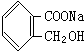 | C�� | H2NCH2COONa | D�� | H2NCH2COOH |
| A�� | �����ڵ���ѹǿ����ʱ����仯 | |
| B�� | 2v����A2��=v����AB�� | |
| C�� | ��λʱ��������2n mol AB��ͬʱ������n mol B2 | |
| D�� | A2��B2��AB�ķ�Ӧ����֮��Ϊ1��1��2 |
| A�� | C12H20O6 | B�� | C15H26O6 | C�� | C18H32O6 | D�� | C21H38O6 |
 �����£���Cl2����ͨ��ˮ�������ͣ�Ȼ�������еμ�0.1mol/LNaOH��Һ������ʵ���������Һ��pH�仯������ͼ��ʾ�������Ǵ�����ֽ⣬����������ȷ���ǣ�������
�����£���Cl2����ͨ��ˮ�������ͣ�Ȼ�������еμ�0.1mol/LNaOH��Һ������ʵ���������Һ��pH�仯������ͼ��ʾ�������Ǵ�����ֽ⣬����������ȷ���ǣ�������| A�� | ʵ���������pH��ֽ�ⶨ��Һ��pH | |
| B�� | a��b�Σ���Һ�� $\frac{c��{H}^{+}��}{c��O{H}^{-}��}$ ��С | |
| C�� | b���Ӧ��Һ�У�c��Cl-����c��H+����c��HClO����c��ClO-����c��OH-�� | |
| D�� | c���Ӧ��Һ�У�c��Na+��=c��Cl-��+c��ClO-�� |
 ��NH4+ ��O2-���������������ͬ���ǣ�������
��NH4+ ��O2-���������������ͬ���ǣ�������| A�� | �ڢۢ� | B�� | �٢ڢۢ� | C�� | �٢ڢ� | D�� | �٢ۢ� |