��Ŀ����
2���絼���Ǻ����������Һ����������С����������������Һ�絼�ʱ仯����ȷ���ζ���Ӧ���յ㣮��0.1mol/L KOH��Һ�ֱ�ζ������Ϊ20mL��Ũ�Ⱦ�Ϊ0.1mol/L��HCl��CH3COOH��Һ�ζ�������ͼ��ʾ�������Һ����仯���Բ��ƣ��������ж���ȷ���ǣ�������
| A�� | ���ߢڴ���0.1mol/L KOH��Һ�ζ�CH3COOH��Һ�ĵζ����� | |
| B�� | ����ͬ�¶��£�P��ˮ����̶ȴ���M�� | |
| C�� | ��M�����Һ���У�c��CH3COO-��+c��OH-��-c��H+��=0.1mol/L | |
| D�� | ��N�����Һ���У�c��K+����c��OH-����c��CH3COO-����c��H+�� |
���� A����Һ��������������Ũ�ȳ����ȣ�CH3COOH��������ʣ���Һ������Ũ�Ƚ�С������KOH����Һ������Ũ��������Һ��������ǿ��
B����������ˮ���룬���������ӵ��δٽ�ˮ���룻
C���κε������Һ�ж����ڵ���غ㣬���ݵ���غ��жϣ�
D��N��ʱ�������Һ��Ϊ�����ʵ���Ũ�ȵ�KOH��CH3COOK����Һ�ʼ��ԣ�CH3COO-ˮ��̶Ƚ�С����������غ��ж�
��� A����Һ��������������Ũ�ȳ����ȣ�CH3COOH��������ʣ���Һ������Ũ�Ƚ�С������KOH����Һ������Ũ��������Һ��������ǿ��HCl��ǿ����ʣ�����KOH��Һ���룬��Һ�����������Һ������Ũ�ȼ�С����Һ������������������ȫ��Ӧʽ����Ũ����С����������KOH��Һ������Ũ��������Һ����������ǿ������ͼ֪�����ߢڴ���0.1 mol/L KOH��Һ�ζ�HC1��Һ�ĵζ����ߣ����ߢٴ���0.1 mol/LKOH��Һ�ζ�CH3COOH��Һ�ĵζ����ߣ���A����
B����������ˮ���룬���������ӵ��δٽ�ˮ���룬P������ΪNaCl��M������Ϊ�����ƴٽ�ˮ���룬��������ͬ�¶��£�P��ˮ�����c��H+��С��M��ˮ����ij̶ȣ���B����
C���κε������Һ�ж����ڵ���غ㣬��M�����Һ�У����ݵ���غ��c��CH3COO-��+c��OH-��-c��H+��=c��K+��=0.05mol/L����C����
D��N��ʱ�������Һ��Ϊ�����ʵ���Ũ�ȵ�KOH��CH3COOK����Һ�ʼ��ԣ�CH3COO-ˮ��̶Ƚ�С����������غ������Ũ�ȴ�С˳����c��K+����c��OH-����c��CH3COO-����c��H+������D��ȷ��
��ѡD��
���� �����Ե絼��Ϊ���忼������Ũ�ȴ�С�Ƚϡ�����ˮ���֪ʶ�㣬��ȷ�����Һ�����ʼ������ʡ���Һ������ǿ��Ӱ�������ǽⱾ��ؼ����״�ѡ����C��ע��A����Һ�����ԭ��2��������Ũ�Ƚ�Ϊԭ��һ�룬��Ŀ�Ѷ��еȣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| A�� | SO2��NxOy���������������� | |
| B�� | �����ڽ��壬�ܲ��������ЧӦ | |
| C�� | �ؽ������ӿɵ��µ����ʱ��� | |
| D�� | ����β���Ĵ����ŷ������������������Ϊ����֮һ |

| A�� | ��Ũ�����ͭ��ȡ�������� | B�� | ��п����ϡ���ᷴӦ��ȡ���� | ||
| C�� | ���Ȼ�����������Ʒ�Ӧ��ȡ���� | D�� | ��ͭ��Ũ���ᷴӦ��ȡ�������� |
| A�� | �ױ������Ȼ�̼ | B�� | �����������ȼ��� | C�� | ��ϩ���� | D�� | �ƾ����� |

| A�� | A��B�ķ�Ӧ���ڼӳɷ�Ӧ | B�� | B��C�ķ�Ӧ����������Ӧ | ||
| C�� | C��D�ķ�Ӧ������ȥ��Ӧ | D�� | D��E�ķ�Ӧ���ڼӳɷ�Ӧ |
| A�� | ��Ӧ���ʿɺ�����ѧ��Ӧ�Ŀ��� | |
| B�� | ������Ӧ���ʵ���Ҫ�����Ƿ�Ӧ������� | |
| C�� | ����Ӧ��Ũ�ȡ���߷�Ӧ���¶ȶ�������Ӧ���� | |
| D�� | ����ͬһ��Ӧ�������ò�ͬ���ʱ�ʾ��ѧ��Ӧ����ʱ��������ֵ����ͬ�� |
 ����ijЩϸ����������������ã�����ʹ��ʯ�еĽ�����ˮ��Һ���ܽ��������������������˾������ÿ����е�����������Һ�н���������Ҫ�ɷ�ΪFeS2������ΪFe2��SO4��3����ʹ��Һ������ǿ���������ͼ��
����ijЩϸ����������������ã�����ʹ��ʯ�еĽ�����ˮ��Һ���ܽ��������������������˾������ÿ����е�����������Һ�н���������Ҫ�ɷ�ΪFeS2������ΪFe2��SO4��3����ʹ��Һ������ǿ���������ͼ�� �ķе��
�ķе��  �ߣ�ԭ����
�ߣ�ԭ����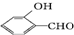 �γɷ������������
�γɷ������������ �γɷ��Ӽ���������Ӽ����ʹ���Ӽ�����������
�γɷ��Ӽ���������Ӽ����ʹ���Ӽ����������� Ϊ�ϳ�ij��Һ�����ϵ��м���M������������²�ͬ�ĺϳ�;��
Ϊ�ϳ�ij��Һ�����ϵ��м���M������������²�ͬ�ĺϳ�;��
 ����C��B��Ӧ����Ϊ�ӳɷ�Ӧ��
����C��B��Ӧ����Ϊ�ӳɷ�Ӧ�� ��
�� ��д�ṹ��ʽ�����ֻ�Ϊͬ���칹���������ɣ�
��д�ṹ��ʽ�����ֻ�Ϊͬ���칹���������ɣ� +NaOH$��_{��}^{ˮ}$
+NaOH$��_{��}^{ˮ}$ +NaCl��
+NaCl�� ��
��