��Ŀ����
 ��������ƵĴ��ȿ��õζ������вⶨ��ԭ���ǣ�2S2O32-+I2��S4O62-+2I-
��������ƵĴ��ȿ��õζ������вⶨ��ԭ���ǣ�2S2O32-+I2��S4O62-+2I-��1������100mL0.0500mol/L I2��Һ������Ҫ��������
a��100mL����ƿ b����Ͳ c���ձ� d��������
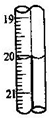
�ζ��ܱ���ʹ���¶ȣ�20�棻�ζ��ܵ���С�̶�Ϊ
��2��ȡ2.500g�����ʵ�Na2S2O3?5H2O�������50mL��Һ��ÿ��ȡ10.00mL������ƿ���2�ε���Ϊָʾ��������0.0500mol/L I2��Һ�ζ���ʵ���������£���3�γ�����Ϊ 0.00���յ������ͼ�� ���ʲ��μӷ�Ӧ����
| ��� | 1 | 2 | 3 |
| ����I2��Һ�����/mL | 19.98 | 20.02 |
��3�������ʵ����ƫ�͵IJ�����
a���ζ�ʱ�ζ����е�Һ�������ƿ��
b����ƿ������ˮϴ��������װ�������Һ
c��δ�ñ�Һ��ϴ�ζ���
d����ʱ��Һ������ƿ�⣮
���㣺�к͵ζ�
ר�⣺ʵ����
��������1����������һ�������ʵ���Ũ����Һ��ʵ����������𣻵ζ��ܵ���С�̶�Ϊ0.1mL��
��2���ٴﵽ�ζ��յ�ʱ��ɫͻ����30s����ɫ��
���ȸ��ݵζ����ĵı�Һ����������ƽ�����������Cr2O72-+6I-+14H+=2Cr3++3I2+7H2O��I2+2S2O32-=2I-+S4O62- ��֪������Cr2O72-��6S2O32-���Դ˼��㣮
��3��a���ζ�ʱ�ζ����е�Һ�������ƿ�⣬���±�Һ�����ƫ��
b����ƿ������ˮϴ��������װ�������Һ������Һ�����ʵ������䣬��Һ�������Ӱ�죻
c��δ�ñ�Һ��ϴ�ζ��ܣ���Һ�����ʵ���Ũ��ƫС�����±�Һ�����ƫ��
d����ʱ��Һ������ƿ�⣬����Һ�����ʵ���ƫС�����±�Һ�����ƫС��
��2���ٴﵽ�ζ��յ�ʱ��ɫͻ����30s����ɫ��
���ȸ��ݵζ����ĵı�Һ����������ƽ�����������Cr2O72-+6I-+14H+=2Cr3++3I2+7H2O��I2+2S2O32-=2I-+S4O62- ��֪������Cr2O72-��6S2O32-���Դ˼��㣮
��3��a���ζ�ʱ�ζ����е�Һ�������ƿ�⣬���±�Һ�����ƫ��
b����ƿ������ˮϴ��������װ�������Һ������Һ�����ʵ������䣬��Һ�������Ӱ�죻
c��δ�ñ�Һ��ϴ�ζ��ܣ���Һ�����ʵ���Ũ��ƫС�����±�Һ�����ƫ��
d����ʱ��Һ������ƿ�⣬����Һ�����ʵ���ƫС�����±�Һ�����ƫС��
���
�⣺��1��������һ�������ʵ���Ũ����Һʱ��һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬���ò��������裬�����ܽ⣮��ȴ��ת�Ƶ�100mL����ƿ�У����ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�����������������ƽ���ձ�����������100mL����ƿ����ͷ�ιܡ�ҩ�ף�����Ҫ��Ͳ���ζ��ܵ���С�̶�Ϊ0.1mL��
�ʴ�Ϊ��b��0.1��
��2���ٴﵽ�ζ��յ�ʱ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ȥ��
�ʴ�Ϊ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ȥ��
��2�����ĵ�I2��Һ�����ƽ��ֵΪ20.00mL����Cr2O72-+6I-+14H+=2Cr3++3I2+7H2O��I2+2S2O32-=2I-+S4O62-�������Cr2O72-��6S2O32-��
1 6
n 0.0500mol/L��0.020L
n=
mol
250ml��Һ�к���Cr2O72-���ʵ���Ϊ
mol��
=
mol��
���ò�Ʒ���ظ���ش���=
��100%=98.00%
�ʴ�Ϊ��98.00%��
��3��a���ζ�ʱ�ζ����е�Һ�������ƿ�⣬���±�Һ�����ƫ��ʵ����ƫ��a����
b����ƿ������ˮϴ��������װ�������Һ������Һ�����ʵ������䣬��Һ�������Ӱ�죬ʵ������Ӱ�죬��b����
c��δ�ñ�Һ��ϴ�ζ��ܣ���Һ�����ʵ���Ũ��ƫС�����±�Һ�����ƫ��ʵ����ƫ��c����
d����ʱ��Һ������ƿ�⣬����Һ�����ʵ���ƫС�����±�Һ�����ƫС��ʵ����ƫС����d��ȷ��
��ѡ��d��
�ʴ�Ϊ��b��0.1��
��2���ٴﵽ�ζ��յ�ʱ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ȥ��
�ʴ�Ϊ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ȥ��
��2�����ĵ�I2��Һ�����ƽ��ֵΪ20.00mL����Cr2O72-+6I-+14H+=2Cr3++3I2+7H2O��I2+2S2O32-=2I-+S4O62-�������Cr2O72-��6S2O32-��
1 6
n 0.0500mol/L��0.020L
n=
| 1 |
| 600 |
250ml��Һ�к���Cr2O72-���ʵ���Ϊ
| 1 |
| 600 |
| 50 |
| 10 |
| 1 |
| 120 |
���ò�Ʒ���ظ���ش���=
| ||
| 2.500g |
�ʴ�Ϊ��98.00%��
��3��a���ζ�ʱ�ζ����е�Һ�������ƿ�⣬���±�Һ�����ƫ��ʵ����ƫ��a����
b����ƿ������ˮϴ��������װ�������Һ������Һ�����ʵ������䣬��Һ�������Ӱ�죬ʵ������Ӱ�죬��b����
c��δ�ñ�Һ��ϴ�ζ��ܣ���Һ�����ʵ���Ũ��ƫС�����±�Һ�����ƫ��ʵ����ƫ��c����
d����ʱ��Һ������ƿ�⣬����Һ�����ʵ���ƫС�����±�Һ�����ƫС��ʵ����ƫС����d��ȷ��
��ѡ��d��
���������⿼���˵ζ����������������������㡢���������Ĺ��죬��Ŀ�Ѷ��еȣ�ע�����յζ������������ܹ�����ָʾ���ص㼰��Ӧԭ����ȷ�жϵζ��յ㣮

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
������Һ�ͽ������ķ����ǣ�������
| A���۲���� | B������ |
| C�����ú�۲� | D�������ЧӦ |
����˵����ȷ���ǣ�������
| A����ij��Һ�м���Ba��NO3��2��Һ�������ɫ��������ԭ��Һ��һ������SO42- |
| B����ijϡ��Һ�м���Ba��NO3��2��Һ�������������ٵ��뼸�������ữ��AgNO3��Һ��������ɫ������˵��һ�����Ȼ������Һ |
| C����ʢ��H2��С�Թܹܿ����Ͽ����ƾ��ƻ������H2�Ĵ��� |
| D����ȼ�ŵ�ľ���������ܿڣ�ľ��Ϩ��˵����CO2���� |

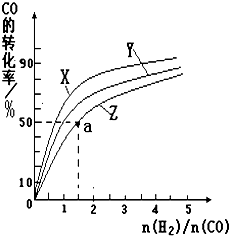 �״���һ������ȼ�ϣ��״�ȼ�ϵ�ؼ�����ʵ��������ҵ����������ҵ��һ����CO��H2Ϊԭ�Ϻϳɼ״����÷�Ӧ���Ȼ�ѧ����ʽΪ��
�״���һ������ȼ�ϣ��״�ȼ�ϵ�ؼ�����ʵ��������ҵ����������ҵ��һ����CO��H2Ϊԭ�Ϻϳɼ״����÷�Ӧ���Ȼ�ѧ����ʽΪ��