��Ŀ����
1�����й��ڹ��Ļ�����˵������ȷ���ǣ�������| A�� | ������Ȼ������Ҫ�Թ����ε���ʽ���ڣ�������ˮ�ࡢ�մɶ����ڹ����β�Ʒ | |
| B�� | �Ŵ����������ϳɷ�֮һ������ɫ�Ĺ���ͭ��BaCuSi2O6���������ȶ���������ɫ������BaO•CuO•2SiO2��ʾ | |
| C�� | ʯӢ��ˮ�������ά����Ҫ�ɷֶ���SiO2��SiO2�����²����κ����ʷ�Ӧ | |
| D�� | �赥��Ӳ�ȴ��۷е�ߣ������õİ뵼����� |
���� A��Si����Ȼ������Ҫ�Ի���̬���ڣ�
B�����ݹ����������������д˳������д��
C���������賣�����ܹ�������ᡢ�������Ʒ�Ӧ��
D���������ԭ�Ӿ��壬�����Խ��ڵ�����뵼��֮�䣮
��� �⣺A��������Ȼ������Ҫ�Թ����ε���ʽ���ڣ�������ˮ�ࡢ�մɶ����ڹ����β�Ʒ����A��ȷ��
B������������������ʽ��ʾʱ����д˳��Ϊ�����ý�������������ý���������������衢ˮ�����Թ���ͭ������������ʽ��ʾ��BaO•CuO•2SiO2����B��ȷ��
C���������賣�����ܹ�������ᡢ�������Ʒ�Ӧ����C����
D���������ԭ�Ӿ��壬Ӳ�ȴ��۷е�ߣ������Խ��ڵ�����뵼��֮�䣬�����õİ뵼����ϣ���D��ȷ��
��ѡ��C��
���� ���⿼��輰�仯��������ʣ���Ŀ�ѶȲ�����Ϥ�輰�������������ǽ���ؼ���ע�⾧�������ԭ�Ӿ��壬��Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
12��A��B��C��D��E��F���ֻ��������A��B��C��D��E���ɶ�����Ԫ����ɣ���ɫ��Ӧ��Ϊ��ɫ��B��C��E��������Ԫ����ɣ�B��C�����Ԫ����ͬ����C��Ħ��������B��80g/mol���ش�
��1�����廯����AΪdz��ɫ��ĩ���û������к��еĻ�ѧ��ΪAC
A�����Ӽ� B�����Թ��ۼ� C���Ǽ��Թ��ۼ� D�����
��2�����ΪB��Fʵ��IJ�������
д��B��ϡH2SO4��Ӧ�����ӷ���ʽS2O32-+2H+=S��+SO2��+H2O��д�����з�Ӧ����ʽFeCl3+3H2O$\frac{\underline{\;\;��\;\;}}{\;}$Fe��OH��3�����壩+3HCl��
��3������6������Mn2+��MnO4-��H+��H2O��X2Y82-��C�к��е������ӣ���XY42-���һ�����ӷ���ʽ����֪Mn2+Ϊ��ԭ�����õ�1mol MnO4-�������������ʵ���Ϊ2.5mol
��4��������D��E�ת��D$?_{CO_{2}+H_{2}O}^{��}$E������D��E•xH2O�Ļ����13.04g�����ȵ���ȫ��Ӧ���������ͨ��ŨH2SO4����3.42g��ʣ������ͨ����ʯ������2.20g����E•xH2O�Ļ�ѧʽΪNa2C03•7H2O��
��1�����廯����AΪdz��ɫ��ĩ���û������к��еĻ�ѧ��ΪAC
A�����Ӽ� B�����Թ��ۼ� C���Ǽ��Թ��ۼ� D�����
��2�����ΪB��Fʵ��IJ�������
| ���ں�B����Һ�м���ϡH2SO4������dz��ɫ���Ǻ�ʹ����ʯ��ˮ����ǵ���ɫ�д̼�����ζ������ |
| ��20mL��ˮ�еμ�F�ı�����Һ1��2mL����Һ��ʺ��ɫ |
| �۽�ʵ��ڵõ��ĺ��ɫҺ��������������գ����յõ�����ɫ���� |
��3������6������Mn2+��MnO4-��H+��H2O��X2Y82-��C�к��е������ӣ���XY42-���һ�����ӷ���ʽ����֪Mn2+Ϊ��ԭ�����õ�1mol MnO4-�������������ʵ���Ϊ2.5mol
��4��������D��E�ת��D$?_{CO_{2}+H_{2}O}^{��}$E������D��E•xH2O�Ļ����13.04g�����ȵ���ȫ��Ӧ���������ͨ��ŨH2SO4����3.42g��ʣ������ͨ����ʯ������2.20g����E•xH2O�Ļ�ѧʽΪNa2C03•7H2O��
9����30mL 1mol•L-1��AlCl3��Һ������Ũ��Ϊ4mol•L-1��NaOH��Һ��������0.78g��ɫ������������NaOH��Һ���������Ϊ��������
| A�� | 27.5 mL | B�� | 17.5 mL | C�� | 15 mL | D�� | 3 mL |
16������ɫ��Һ�У����������ܴ���������ǣ�������
| A�� | NH4+��Na+��S2-��ClO- | B�� | K +��SO42-��OH-��AlO2- | ||
| C�� | K +��Fe3+��Cl-��NO3- | D�� | Ba2+��Na+��OH-��CO32- |
13����NA��ʾ�����ӵ�������ֵ������˵����ȷ���ǣ�������
| A�� | 7.8 g Na2O2��CO2��ȫ��Ӧ��ת�Ƶ�����Ϊ0.2NA | |
| B�� | 4 g H2O2��ȫ�ֽ�ת�Ƶ���0.2NA | |
| C�� | 2.4 g Mg������O2������N2��ȫ��Ӧ��ת�Ƶ���������0.2NA | |
| D�� | 5.6 g Fe��Cl2��ȫ��Ӧ��ת�Ƶ�����Ϊ0.2NA |
10������������ȷ���ǣ�������
| A�� | ͬ��ͬѹ�£���ͬ��������ʣ������ʵ�����Ȼ��� | |
| B�� | �κ������£������ʵ�������ϩ��C2H4����һ����̼�����ķ�������Ȼ��� | |
| C�� | 1 Lһ����̼����һ����1 L����������С | |
| D�� | ͬ��ͬѹ�£�����������������ķ�����һ����� |

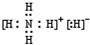 ��
��