��Ŀ����
10��)ijѧ������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һʱ��ѡ�������ָʾ��������д���пհף�
��1���ñ�������ζ������NaOH��Һʱ����������ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�ע��________��ֱ�������һ���������Һ�ɻ�ɫ��Ϊ��ɫ����____________Ϊֹ��
��2�����в����п���ʹ����NaOH��Һ��Ũ����ֵƫ�͵���________(����ĸ���)��
| A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע������� |
| B���ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и��� |
| C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ |
| D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ��� |
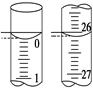
��4��ijѧ������3��ʵ��ֱ��¼�й��������±���
| �ζ����� | ����NaOH��Һ�����/mL | 0.100 0 mol��L��1��������/mL | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
| ��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
| �ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
| ������ | 25.00 | 0.22 | 26.31 | 26.09 |
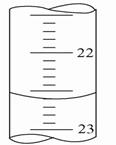
22 ��1����ƿ����Һ��ɫ�仯 �ڰ�����ڲ���ɫ ��2��D ��3�� 26.10
��4��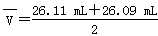 ��26.10 mL��
��26.10 mL��
c(NaOH)��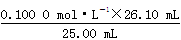 ��0.104 4 mol��L��1
��0.104 4 mol��L��1
���������������1���۾��۲������ƿ��ָʾ����ɫ�ı仯�������ǵζ�����Һ�������仯���жϵζ��յ�ʱ��ע����ָʾ����ɫ�����仯������ڲ��ٸ�ԭ��ֹͣ�ζ�����Ϊ��ƿ����Һ��ɫ�仯 �ڰ�����ڲ���ɫ��
��2��A.����δ�ñ�Һ��ϴ��������������������ʵ����Ҫ�ⶨ������������Һ��Ũ��ƫ�ߡ�B.��Ӱ�죻C.��ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ,ֱ�ӵ��¶�����ʵ��ֵƫ��ʹ�ⶨ������������Һ��Ũ��ƫ�ߣ�D.��ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���,���¶�����ʵ����ƫС��ʹ�ⶨ������������Һ��Ũ��ƫ�ͣ�ѡD��
��3����ͼ��֪���ζ�ǰ�̶�0��00���ζ���̶�26.10����Һ���26.10��
��4���ڶ������ݣ����̫����ȥ��ȡ��һ�κ͵ڶ������������ƽ��ֵ��V="(26.11+26.09)/2=" 26.10(mL)����Ũ��Ϊ0.100 0 mol/L����������������Һ�����Ϊ25.00 mL���������������Һ�����ʵ���Ũ��c(NaOH)=c(����)��V(����) /V(NaOH)="0.104" 4 mol/L��
���㣺��������к͵ζ���

���ڻ�ѧ���������У���ȷ����
| A�����ӻ�������ܺ����ۼ� |
| B�����ۻ�������ܺ����Ӽ� |
| C�����ӻ�������ֻ�����Ӽ� |
| D�����ӻ�������һ�����н������� |
���������м��������������ʵ���Һ�������գ���Һ����400�棩���Եõ�ԭ���ʹ������
| A��AlCl3 | B��NaHCO3 | C��MgSO4 | D��KMnO4 |
��10�֣�ʵ��������һδ֪Ũ�ȵ�ϡ���ᣬijѧ��ʵ�����н��вⶨ����Ũ�ȵ�ʵ�飬�����������ա�
��1������100 mL 0.10 mol��L-1 NaOH����Һ��
����Ҫ��������:������������ܽ��(��ȴ��)ת�ơ�ϴ��(����ϴ��Һ��������ƿ)�����ݡ�ҡ�ȡ������ƺõ���Һ�����Լ�ƿ�����ϱ�ǩ��
��������ƽ���� g�������ƹ��塣
��2��ȡ20.00 mL�������������ƿ�У����μ�2~3�η�̪��ָʾ�������Լ����Ƶ�NaOH����Һ���еζ����ظ������ζ�����2~3��,��¼�������¡�
| ʵ���� | NaOH��Һ��Ũ��/(mol��L-1) | �ζ����ʱ,NaOH��Һ��������/mL | ������������/mL |
| 1 | 0.10 | 22.58 | 20.00 |
| 2 | 0.10 | 22.72 | 20.00 |
| 3 | 0.10 | 22.80 | 20.00 |
�ڸ����������ݣ��ɼ�����������Ũ��ԼΪ (������λ��Ч����)��
����ȥ��ʽ�ζ��������ݵķ���Ӧ������ͼ��ʾ�����е� ��Ȼ�����ἷѹ������ʹ���첿�ֳ�����Һ��

��������ʵ���У����в���(����������ȷ)����ɲⶨ���ƫ�ߵ��� ��
A.�ζ��յ����ʱ���Ӷ���
B.��ʽ�ζ���ʹ��ǰ,ˮϴ��δ�ô���������ϴ
C.��ƿˮϴ��δ����
D.��ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ
���������У���Ͷ�����˽����������䡱���̣��������̲صķḻ��Դͨ���ܵ����͵�����������������ָ�ġ�����������Ҫ�ɷ���
| A��CH4 | B��CO | C��H2 | D��NH3 |
����������ˮ�����ѵ���������ӵ���
| A��CH3COOH | B��C2H5OH | C��H2O | D��C6H5OH |