��Ŀ����
12�� ͭ��һ����Ҫ����ɫ��������������;Խ��Խ�㷺����ش��������⣺
ͭ��һ����Ҫ����ɫ��������������;Խ��Խ�㷺����ش��������⣺��1���������ֻ������к�ͭ����ߵ���C������ĸ��
A��Cu5FeS4 B��CuFeS2 C��Cu2S D��Cu2��OH��2CO3
��2��2014���ҹ�����ͭ����796��֣���ȫ���ɺ�Cu2S��������Ϊ32%��ͭ��ʯұ���õ�������Ҫͭ��ʯ����Ϊ3109.4��֣�������һλС����
��3��������ͭ�γ���������������ͭ�������KOH��Һ�м���һ������CuSO4��Һ���ټ���һ�����Ļ�ԭ��--�£�N2H4�������Ȳ������¶���90�棬����һ�ֶԻ�������Ⱦ�����壬��Ӧ��ȫ���룬ϴ�ӣ���ո���õ�����������ͭ���壨Cu2O����
�ٸ��Ʊ����̵ķ�Ӧ����ʽΪ4CuSO4+N2H4+8KOH$\frac{\underline{\;90��\;}}{\;}$2Cu2O+N2��+4K2SO4+6H2O��
�ڹ�ҵ�ϳ��õĹ�Һ�����豸��AC������ĸ��
A�����Ļ� B�������� C����ʽѹ�˻� D����Ӧ��
��4���ҹ���������ͭ�����վ�տ�����кܸߵ�������ֵ����ʷ��ֵ������������ͭ������ܵ�������ʴ����ͼ����ͭ���ڳ�ʪ�����з����绯ѧ��ʴ��ԭ��ʾ��ͼ��
�ٸ�ʴ�����У�������c���a����b����c������������Ӧ����ʽΪO2+2H2O+4e-�T4OH-��
�ڻ����е�Cl-��ɢ���ڣ�����������������������ɶ��״��Cu2��OH��3Cl�������ӷ���ʽΪ2Cu2++3OH-+Cl-=Cu2��OH��3Cl����
���� ��1����ͭ��=$\frac{����ͭ�����ԭ������}{���ʵĺ�ͭ��}$��
��2�����ݻ�ѧʽCu2S����ͭԪ�ص�����������������һ��������Cu2S������ͭԪ�ص�������������ijԪ�ص��������������Ǹ�Ԫ�ص�������������ʵ�Ԫ��������֮�ȣ�
��3�������������֪��������������ͭ��N2H4����������ԭ��Ӧ����Cu2O��N2��
�ڹ�ҵ�ϳ��õĹ�Һ�����豸�����Ļ��Ϳ�ʽѹ�˻���
��4���ٸ���ͼ֪�������õ����������������ӡ�Cuʧ��������ͭ���ӣ�����������ʴ����Cu��������
��Cl-��ɢ���ڣ�����������Ӧ���������Ӧ�����������ɶ��״��Cu2��OH��3Cl������������ͭ���ӡ��������������������ӣ����Ը����ӷ�ӦΪ�����ӡ�ͭ���Ӻ����������ӷ�Ӧ����Cu2��OH��3Cl������
��� �⣺��1��A��Cu5FeS4�к�ͭ��Ϊ$\frac{320}{504}$=0.63��
B��CuFeS2�к�ͭ��Ϊ$\frac{64}{184}$=0.35��
C��ͭ��Ϊ$\frac{128}{160}$=0.8��
D��Cu2��OH��2CO3�к�ͭ��Ϊ$\frac{128}{221}$=0.58��
�ʴ�Ϊ��C��
��2��Cu2S��ͭԪ�ص���������=$\frac{64��2}{160}$��100%=80%����X�ֺ�Cu2S 32%��ͭ��ʯ�к�ͭԪ������=Xt��80%��32%=796��t�����X=3109.4��֣�
�ʴ�Ϊ��3109.4��
��3�����������֪��������������ͭ��N2H4����������ԭ��Ӧ����Ӧ�Ļ�ѧ����ʽΪ4CuSO4+N2H4+8KOH$\frac{\underline{\;90��\;}}{\;}$2Cu2O+N2��+4K2SO4+6H2O��
�ʴ�Ϊ��4CuSO4+N2H4+8KOH$\frac{\underline{\;90��\;}}{\;}$2Cu2O+N2��+4K2SO4+6H2O��
�ڹ�ҵ�ϳ��õĹ�Һ�����豸�����Ļ��Ϳ�ʽѹ�˻����ʴ�Ϊ��AC��
��4���ٸ���ͼ֪�������õ����������������ӡ�Cuʧ��������ͭ���ӣ�����������ʴ����Cu����������c�Ǹ���������������ԭ��Ӧ���缫����ʽΪO2+2H2O+4e-�T4OH-��
�ʴ�Ϊ��c��
��Cl-��ɢ���ڣ�����������Ӧ���������Ӧ�����������ɶ��״��Cu2��OH��3Cl������������ͭ���ӡ��������������������ӣ����Ը����ӷ�ӦΪ�����ӡ�ͭ���Ӻ����������ӷ�Ӧ����Cu2��OH��3Cl���������ӷ���ʽΪ2Cu2++3OH-+Cl-=Cu2��OH��3Cl����
�ʴ�Ϊ��2Cu2++3OH-+Cl-=Cu2��OH��3Cl����
���� ���⿼��Cu���仯��������ʣ�Ϊ��Ƶ���㣬�������ʵ������Լ��绯ѧ��Ӧԭ����Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬��Ŀ�ѶȲ���


����˵������ȷ���ǣ�������
| A�� | ��������SiO2����������Fe��OH��3 | |
| B�� | ʹ��ʯ��ˮʱ��Ҫ����pH����ֹ�������Al��OH��3ת��ΪAlO2- | |
| C�� | �Լ�aѡ�����ᣬ����Һ��õ�CaCl2•6H2O��Ʒ�Ĺ����У������������ֹ��ֽ� | |
| D�� | ���ı�ʵ�鷽��������Һ����ֱ�ӼӰ�ˮ��������ȫ����ȥ����������Һ������Ũ������ȴ�ᾧҲ�ɵõ�����CaCl2•6H2O |
C��s��+H2O��g���TCO��g��+H2��g����H=bkJ•mol-1
H-H��O-H��O=O���ļ��ֱܷ�Ϊ436��462��495kJ•mol-1����bΪ��������
| A�� | +352 | B�� | +132 | C�� | -120 | D�� | -330 |
| A�� |  ������п�����������ᷴӦ | |
| B�� |  ������������Ӧ�е������仯 | |
| C�� |  ��̬�⻯��е� | |
| D�� |  ����Ӧ��Ǵ���Ӧ�����е�������ϵ |
| A�� | HCl | B�� | CH3COONa | C�� | NaOH | D�� | NaCl |
| A�� | һ�����淴Ӧ�ﵽ��ƽ��״̬���������Ӧ�ڸ����������ܴﵽ���� | |
| B�� | ����Ӧ��Ũ�ȣ����������Ӱٷ��������Է�Ӧ�������� | |
| C�� | �ڻ�ѧ��Ӧ�����У��������ʱ仯��ͬʱ��һ�����������仯 | |
| D�� | 100 mL 2 mol/L�������пƬ��Ӧ�������������Ȼ�����Һ����Ӧ���ʲ��� |


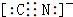 ��
��
