��Ŀ����
17����ǰ���Ͽ�ѧ�ķ�չ����δ����B��N��Ti��Fe������Ҫ�IJ���Ԫ�أ��䵥�ʼ�����������������ж��й㷺��Ӧ�ã���1����̬Fe2+�ĵ����Ų�ʽΪ1S22S22P63S23P63d6��[Ar]3d6��Tiԭ�Ӻ����22���˶�״̬��ͬ�ĵ��ӣ�
��2��BF3������NH3���ӵĿռ�ṹ�ֱ�Ϊƽ���������Ρ������ͣ�BF3��NH3��Ӧ���ɵ�BF3•NH3�����к��еĻ�ѧ�������й��ۼ�����λ������BF3•NH3��Bԭ�ӵ��ӻ���ʽΪsp3��
��3��N��Pͬ���壮��ѧ��Ŀǰ�ϳ���N4���ӣ��÷�����N-N���ļ���Ϊ60�㣻N4�ֽ���ܲ���N2���ͷų������������Ʋ�����;�������ƽ�����ըҩ����д��һ�ּ��ɣ�
��4��������ͭ��Һ�м��������ˮ��������[Cu��NH3��4]2+�����ӣ���֪NF3��NH3������ͬ�Ŀռ乹�ͣ���
NF3������Cu2+�γ������ӣ���ԭ����F�ĵ縺�Ա�N��N-F�ɼ����Ӷ�ƫ��F������NF3�е�ԭ�Ӻ˶���µ��ӶԵ�����������ǿ�������γ���λ����
��5������TiO2��һ��Ӧ�ù㷺�Ĵ����������һ��ʵ����ͼ1��ʾ���������ҵķе����Ը��ڻ�����ף���Ҫԭ���ǻ������ҷ��Ӽ������������������в�ȡsp3�ӻ���ԭ�ӵĵ�һ�������ɴ�С��˳��ΪN��O��C��
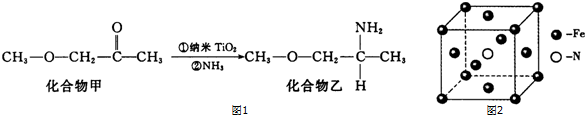
��6�����Ͱ�����640��ɷ����û���Ӧ������֮һ�ľ����ṹ��ͼ2��ʾ��д���÷�Ӧ�Ļ�ѧ����ʽ8Fe+2NH3$\frac{\underline{\;640��\;}}{\;}$2Fe4N+3H2�����þ�����ܶ��Ǧ�g•cm-3�������������Feԭ�Ӽ�ľ���Ϊ$\frac{{\sqrt{2}}}{2}\root{3}{{\frac{238}{{��{N_A}}}}}$cm���������ӵ�������NA��ʾ��
���� ��1����ԭ��ʧȥ�����4s�ܼ�2�����ӣ��γ�Fe2+����������Ų�ʽΪ��1s22s22p63s23p63d5�����Ӳ�ͬ�˶�״̬��ͬ��
��2��NH3�����е�ԭ�Ӻ��йµ��Ӷԣ�BF3������BԪ�ز����µ��Ӷԣ�������ռ乹�Ͳ�ͬ�����ݼ۲���ӶԻ�������ȷ��BF3�ķ��ӿռ乹�ͣ�
��3��N4������P4�ṹ���ƣ�Ϊ�������幹�ͣ�N4������Nԭ���γ�3���Ҽ�������1�Թ¶Ե��ӣ��ӻ������ĿΪ4��ÿ����Ϊ�������Σ�N4�ֽ���ܲ���N2���ͷų����������������������ƽ�����ըҩ��
��4��NF3��N-F�ɼ����Ӷ�ƫ����Fԭ�ӣ�Nԭ���ϵŶԵ�������ͭ�����γ������ӣ�
��5������Ĵ��ڵ��������۷е����ߣ�ͬһ����Ԫ�أ�Ԫ�ص�һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ�
��6�����Ͱ�����640��ɷ����û���Ӧ���������͵����������þ�̯��ȷ���������Ļ�ѧʽ�������¶ȡ���Ӧ���������д����Ӧ����ʽ��
�ɾ������V=$\frac{m}{��}$����������̶���������������ⳤ��������������ⳤ���������Feԭ�Ӽ�ľ��룮
��� �⣺��1����ԭ��ʧȥ�����4s�ܼ�2�����ӣ��γ�Fe2+����������Ų�ʽΪ��1s22s22p63s23p63d6��[Ar]3d6��TiԪ��ԭ�Ӻ�����22�����ӣ�����ԭ�����˶�״̬��ͬ�ĵ��ӹ���22�֣�
�ʴ�Ϊ��1S22S22P63S23P63d6��[Ar]3d6��22��
��2��NH3�����е�ԭ�Ӻ��йµ��Ӷԣ�BF3������BԪ�ز����µ��Ӷԣ�NH3��Nԭ�Ӻ���3���Ҽ���1���µ��Ӷԣ�����NH3Ϊ�������ͣ�BF3��Bԭ�Ӻ���3���Ҽ��Ҳ����µ��Ӷԣ�����BF3Ϊƽ�������ι��ͣ�BF3•NH3�����к��еĻ�ѧ�������й��ۼ�����λ������BF3•NH3��Bԭ�ӵ��Ӷ���Ϊ$\frac{5+3}{2}$�����ӻ���ʽΪsp3��
�ʴ�Ϊ��ƽ���������Σ������ͣ����ۼ�����λ����sp3��
��3��N4������Nԭ���γ�3���Ҽ�������1�Թ¶Ե��ӣ��ӻ������ĿΪ4��Nԭ�Ӳ�ȡsp3�ӻ���ÿ����Ϊ�������Σ�N-N ���ļ���Ϊ60�㣬N4�ֽ���ܲ���N2���ͷų����������������������ƽ�����ըҩ��
�ʴ�Ϊ��60�㣻�������ƽ�����ըҩ��
��4��F�ĵ縺�Դ���NԪ�أ�NF3��N-F�ɼ����Ӷ�ƫ����Fԭ�ӣ�����NF3�е�ԭ�Ӻ˶���µ��ӶԵ�����������ǿ��Nԭ���ϵŶԵ�������ͭ�����γ������ӣ�����NF3������Cu2+�γ������ӣ�
�ʴ�Ϊ��F�ĵ縺�Ա�N��N-F�ɼ����Ӷ�ƫ��F������NF3�е�ԭ�Ӻ˶���µ��ӶԵ�����������ǿ�������γ���λ����
��5������Ĵ��ڵ��������۷е����ߣ����к���������ײ�����������Ի��������۷е���ڼף����γ�sp3�ӻ���ԭ����C��N��OԪ�أ�ͬһ����Ԫ�أ�Ԫ�ص�һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ����Ե�һ������N��O��C��
�ʴ�Ϊ���������ҷ��Ӽ���������N��O��C��
��6���þ�������ԭ�Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4����ԭ�Ӹ�����1�����Ե������Ļ�ѧʽ��Fe4N�����Ͱ�����640��ɷ����û���Ӧ���������͵����������Ը÷�Ӧ����ʽΪ��8Fe+2NH3$\frac{\underline{\;640��\;}}{\;}$2Fe4N+3H2��һ��������Feԭ����ĿΪ8��$\frac{1}{8}$+6$+��\frac{1}{2}$=4��Nԭ����ĿΪ1�������ⳤa=$\root{3}{\frac{m}{��}}$=$\root{3}{\frac{56��4+14}{��{N}_{A}}}$�������������Feԭ�Ӽ�ľ���Ϊ$\frac{\sqrt{2}}{2}a$
=$\frac{{\sqrt{2}}}{2}\root{3}{{\frac{238}{{��{N_A}}}}}$��
�ʴ�Ϊ��8Fe+2NH3$\frac{\underline{\;640��\;}}{\;}$2Fe4N+3H2��$\frac{{\sqrt{2}}}{2}\root{3}{{\frac{238}{{��{N_A}}}}}$��
���� �����Ƕ����ʽṹ�Ŀ��飬�漰���ӽṹ���ӻ�������縺�ԡ���������Ų�ʽ�������ṹ�ȣ�ע��������λ�����йظ���Ѷ��еȣ�

| A�� | ̼���� | B�� | ���� | C�� | ��ʽ�� | D�� | ���� |
| A�� |  ��ʾ�ϳɰ������ȷ�Ӧ�����¶�T1��T2��Ӧ��ƽ�ⳣ��ΪK1��K2���t��T1��T2��K1��K2 | |
| B�� |  ��Ӧ��X��g��+2Y��g��?3Z��g����b�Ĝضȱ�a���ܸ� | |
| C�� |  ���¶��£�����FeCl3�������������b��a�ı仯 | |
| D�� |  ��ˮϡ��pH��ͬ������ʹ��ᣬ���ʾ���ᣬ���ʾ���ᣬ����Һ�����ԣ�c��b��a |

��֪���������Ҫ�ɷ�Ϊ2CaO•3Al2O3•FeO•Fe2O3•10SiO2•nH2O�����������↑ʼ����ʱ����ȫ����ʱ��pH�����ʾ��
| Fe��OH��2 | Fe��OH��3 | Al��OH��3 | |
| ��ʼ����ʱ��pH | 6.3 | 1.9 | 3.4 |
| ��ȫ����ʱ��pH | 8.3 | 3.2 | 4.7 |
��2������Һ���м���H2O2�������ǽ���Һ�е�Fe2+����ΪFe3+����CaCO3������ҺpHԼΪ3.3����Ŀ����ʹFe3+��ȫת��ΪFe��OH��3��������ȥ��
��3����ɫˮ�����Ӽ�����Ҫ�ɷ���Fe2O3��
��4������X�Ļ�ѧʽΪCaSO4•2H2O��
��5������M�ĵ���ʽΪ
 ������Һ���м���CaO����������ˮ��Ӧ���ȣ�����NH3���ܽ�ȣ���дһ������
������Һ���м���CaO����������ˮ��Ӧ���ȣ�����NH3���ܽ�ȣ���дһ��������6���������㷺���ڵ����մɵȹ�ҵ������������̼������Ϊԭ�Ͽ��Ʊ����������û�ѧ����ʽ��ʾ�Ʊ����̣�2Al��OH��3$\frac{\underline{\;\;��\;\;}}{\;}$Al2O3+3H2O��Al2O3+N2+3C$\frac{\underline{\;����\;}}{\;}$2AlN+3CO��
| A�� | BF3 | B�� | NCl3 | C�� | PCl5 | D�� | CHCl3 |



 ��
�� ��
�� ��
��
 �ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£� ��
��