��Ŀ����
Ϊ�˲ⶨ�������ƺ�̼���ƹ�������mg��̼���Ƶ������������ס�����λͬѧ�ֱ���������µ�ʵ�鷽����
��һ����ͬѧ�ķ����ǣ�
����Ʒ�ܽ⣬�ӹ����Ȼ�����Һ������ϴ�ӣ�ȡ������ɣ������ù���ng��
��1���������̼���Ƶ���������Ϊ����m��n��ʾ��
��2����ͬѧ��ѡ�õIJ���������
��3����Ca2+��Ba2+����ʹCO32-������ȫ����ʹ���Ȼ�����Һ���Ȼ�����Һ���õĽ�����и��ߵľ�ȷ�ȣ�ԭ����
��������ͬѧ�ķ�����ͼ��ʾ��

��1��������ͬѧ��ʵ��װ��ͼ��������ʵ������б�������ƽ���г����IJ����У�
�� ���� ����
��2�����ظ���ȷ���������Σ�������ݳ����˽ϴ��ƫ�����Ϊ��Ҫԭ������ǣ�ֻд��������
�� �� ��
��һ����ͬѧ�ķ����ǣ�
����Ʒ�ܽ⣬�ӹ����Ȼ�����Һ������ϴ�ӣ�ȡ������ɣ������ù���ng��
��1���������̼���Ƶ���������Ϊ����m��n��ʾ��
��2����ͬѧ��ѡ�õIJ���������
��3����Ca2+��Ba2+����ʹCO32-������ȫ����ʹ���Ȼ�����Һ���Ȼ�����Һ���õĽ�����и��ߵľ�ȷ�ȣ�ԭ����
��������ͬѧ�ķ�����ͼ��ʾ��

��1��������ͬѧ��ʵ��װ��ͼ��������ʵ������б�������ƽ���г����IJ����У�
��
��2�����ظ���ȷ���������Σ�������ݳ����˽ϴ��ƫ�����Ϊ��Ҫԭ������ǣ�ֻд��������
���㣺̽�����ʵ���ɻ�������ʵĺ���
ר�⣺ʵ��̽�������ݴ�����
��������һ����1���������̼���Ƶ���������=
��100%������������Ϊmg��̼���Ƶ������ɸ���̼�ᱵ������ng�������
��2����ͬѧ��Ҫ����Ϊ�ܽ⡢���ˣ�����ѡ�õIJ����������ձ�����������©����
��3�������Ȼ��ƵĻ�����Ӧ���ɵ�������������ˮ����ʹ���������������̼��Ƶ���Է�����С��̼�ᱵ���ᵼ�³������ƫ��
��������1��������ͬѧ��ʵ�鷽�����ݼ�ʯ�ҵ����ؼ�Ϊ������̼������������̼���Ƶ��������ݴ��ж���Ҫ���еij���������
��2��û�г�ȥװ���еĿ����������к��ж�����̼��Ӱ��ⶨ�������Ӧ���װ���л��в����Ķ�����̼���壬��ʯ�һ�������������е�ˮ�Ͷ�����̼�ݴ˷�����
| ̼���Ƶ����� |
| ��Ʒ���� |
��2����ͬѧ��Ҫ����Ϊ�ܽ⡢���ˣ�����ѡ�õIJ����������ձ�����������©����
��3�������Ȼ��ƵĻ�����Ӧ���ɵ�������������ˮ����ʹ���������������̼��Ƶ���Է�����С��̼�ᱵ���ᵼ�³������ƫ��
��������1��������ͬѧ��ʵ�鷽�����ݼ�ʯ�ҵ����ؼ�Ϊ������̼������������̼���Ƶ��������ݴ��ж���Ҫ���еij���������
��2��û�г�ȥװ���еĿ����������к��ж�����̼��Ӱ��ⶨ�������Ӧ���װ���л��в����Ķ�����̼���壬��ʯ�һ�������������е�ˮ�Ͷ�����̼�ݴ˷�����
���
�⣺��һ����1����̼���Ƶ�����Ϊx
Na2CO3+BaCl2=BaCO3��+2NaCl
106 197
x ng
x=
=
g��
�������̼���Ƶ���������Ϊ��
��100%=
��
�ʴ�Ϊ��
��
��2����ͬѧ��Ҫ����Ϊ�ܽ⡢���ˣ�����ѡ�õIJ����������ձ�����������©�����ʴ�Ϊ���ձ�����������©����
��3�������Ȼ��ƣ���Ӧ���ɵ�������������ˮ����ʹ���������������̼��Ƶ���Է�����С��̼�ᱵ���ᵼ�³������ƫ��
�ʴ�Ϊ��Ca��OH��2����ˮ������Ӱ�����������BaCl2��CaCl2����Է������������ij��������������С��
��������1�����ݼ�ʯ�ҵ����ؼ�Ϊ������̼������������̼���Ƶ�����������̼���Ƶ���������̼���Ƶ���������������ʵ��ʱҪ�������³���������ʵ��ǰ�����������Ʒ��������ʵ��ǰ������ʯ�������ܵ���������ʵ��������ʯ�������ܵ���������
�ʴ�Ϊ����ʵ��ǰ�����������Ʒ����������ʵ��ǰ������ʯ�������ܵ�����������ʵ��������ʯ�������ܵ���������
��2���÷�����û�г�ȥװ���еĿ����������к��ж�����̼��Ӱ��ⶨ��������ⷴӦ���װ����Ҳ�в����Ķ�����̼���壻��ʯ��Ҳ��������������е�ˮ�Ͷ�����̼�����Խ�����ݳ����˽ϴ��ƫ�
�ʴ�Ϊ��װ����ԭ�еĿ����еĶ�����̼����û���ų���Ҳ����ʯ�����գ���Ӧ��ɺ�װ���еĶ�����̼û��ȫ������ʯ�����գ������е�ˮ�����Ͷ�����̼����ʯ�����գ�
Na2CO3+BaCl2=BaCO3��+2NaCl
106 197
x ng
x=
| 106��ng |
| 197 |
| 106n |
| 197 |
�������̼���Ƶ���������Ϊ��
| ||
| m |
| 106n |
| 197m |
�ʴ�Ϊ��
| 106n |
| 197m |
��2����ͬѧ��Ҫ����Ϊ�ܽ⡢���ˣ�����ѡ�õIJ����������ձ�����������©�����ʴ�Ϊ���ձ�����������©����
��3�������Ȼ��ƣ���Ӧ���ɵ�������������ˮ����ʹ���������������̼��Ƶ���Է�����С��̼�ᱵ���ᵼ�³������ƫ��
�ʴ�Ϊ��Ca��OH��2����ˮ������Ӱ�����������BaCl2��CaCl2����Է������������ij��������������С��
��������1�����ݼ�ʯ�ҵ����ؼ�Ϊ������̼������������̼���Ƶ�����������̼���Ƶ���������̼���Ƶ���������������ʵ��ʱҪ�������³���������ʵ��ǰ�����������Ʒ��������ʵ��ǰ������ʯ�������ܵ���������ʵ��������ʯ�������ܵ���������
�ʴ�Ϊ����ʵ��ǰ�����������Ʒ����������ʵ��ǰ������ʯ�������ܵ�����������ʵ��������ʯ�������ܵ���������
��2���÷�����û�г�ȥװ���еĿ����������к��ж�����̼��Ӱ��ⶨ��������ⷴӦ���װ����Ҳ�в����Ķ�����̼���壻��ʯ��Ҳ��������������е�ˮ�Ͷ�����̼�����Խ�����ݳ����˽ϴ��ƫ�
�ʴ�Ϊ��װ����ԭ�еĿ����еĶ�����̼����û���ų���Ҳ����ʯ�����գ���Ӧ��ɺ�װ���еĶ�����̼û��ȫ������ʯ�����գ������е�ˮ�����Ͷ�����̼����ʯ�����գ�
���������⿼����̼���ơ�̼�����Ƶ����ʡ���ѧʵ�鷽������������ۣ���Ŀ�Ѷ��еȣ�ע������̼���ơ�̼�����Ƶ����ʣ���ȷʵ�鷽������������۷������������������ѧ���ķ������������������Ӧ����ѧ֪ʶ��������

��ϰ��ϵ�д�
�����Ŀ
�����йع�ҵ������������ȷ���ǣ�������
| A���ϳɰ����������н�NH3Һ�����룬�ή�ͷ�Ӧ���ʣ������N2��H2��ת���� |
| B���Ӻ�ˮ����þ�����У��������MgO���Ʊ�Mg |
| C����⾫��ͭʱ��ͬһʱ���������ܽ����������������������������С |
| D����ⱥ��ʳ��ˮ���ռ�������ӽ���Ĥ�����ɷ�ֹ�����Ҳ�����Cl2���������� |
��1mol SO2��1mol O2ͨ��һ�ݻ�������ܱ������У���һ���¶Ⱥʹ��������£�������Ӧ��2SO2��g��+O2��g��?2SO3��g������Ӧ�ﵽƽ��ʱSO3Ϊ0.3mol������ʱ����0.5mol O2��0.5mol SO2�������´ﵽƽ���SO3�����ʵ�����������
| A������0.3 mol |
| B������0.15 mol |
| C����0.15 mol |
| D������0.15 mol����0.3 mol |
����˵����ȷ���ǣ�������
| A���а�ˮ���Լ���MgCl2��Һ��AlCl3��Һ |
| B�������ó����ʯ��ˮ����SO2��CO2 |
| C��SO2��ʹFeCl3��KMnO4ˮ��Һ��ɫ |
| D����������CuSO4��Һ��Ӧ����������Cu |
���������У������ڴ�����ǣ�������
| A��C3H7OH |
| B��C6H5OH |
| C��C6H5CH2OH |
| D������ |


 ��
�� +CH3COOH
+CH3COOH +H2O
+H2O ע�����η������������൱�ڶ����������ڷе���̫��Ļ����ķ���
ע�����η������������൱�ڶ����������ڷе���̫��Ļ����ķ���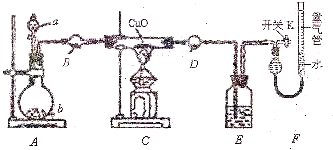
 �����ü���ʽ��ʾΪ
�����ü���ʽ��ʾΪ ����������X��Ϊͬ���칹�����
����������X��Ϊͬ���칹�����

 ����ϵͳ������������Ϊ
����ϵͳ������������Ϊ