��Ŀ����
�ڻ���ƽ�����װ��ǿ��ԭ���£�N2H4����ǿ��������H2O2���������ǻ��ʱ��������������N2��ˮ���������ų������ȣ���֪0.4molҺ̬�º�����H2O2��Ӧ�����ɵ�����ˮ�������ų�256.65KJ��������
��1��д���÷�Ӧ���Ȼ�ѧ����ʽ ��
��2����֪H2O��l���TH2O��g������H=+44kJ?mol-1����16gҺ̬ˮ����ʱ���ų��������� KJ��
��1��д���÷�Ӧ���Ȼ�ѧ����ʽ
��2����֪H2O��l���TH2O��g������H=+44kJ?mol-1����16gҺ̬ˮ����ʱ���ų���������
���㣺�Ȼ�ѧ����ʽ,�йط�Ӧ�ȵļ���
ר�⣺��ѧ��Ӧ�е������仯
��������1������1molҺ̬��������Һ̬˫��ˮ��Ӧʱ�ų�����������д����Ӧ���Ȼ�ѧ����ʽ��
��2�����ݸ�˹������д���㣻
��2�����ݸ�˹������д���㣻
���
�⣺��1��0.4molҺ̬�º�����H2O2��Ӧ�����ɵ�����ˮ�������ų�256.65kJ��������1molҺ̬��������Һ̬˫��ˮ��Ӧʱ�ų�������641.625kJ�����Ȼ�ѧ����ʽΪ��N2H4��l��+2H2O2��l���TN2��g��+4H2O��g����H=-641.625 kJ?mol-1��
�ʴ�Ϊ��N2H4��l��+2H2O2��l���TN2��g��+4H2O��g����H=-641.625 kJ?mol-1��
��2��H2O��l���TH2O��g����H=+44kJ?mol-1��16gҺ̬ˮ����ʱ�ų�������=
=39.1kJ���ʴ�Ϊ��39.1��
�ʴ�Ϊ��N2H4��l��+2H2O2��l���TN2��g��+4H2O��g����H=-641.625 kJ?mol-1��
��2��H2O��l���TH2O��g����H=+44kJ?mol-1��16gҺ̬ˮ����ʱ�ų�������=
| 16g��44KJ |
| 18g |
������������Ҫ�������Ȼ�ѧ����ʽ����д����Ҫע����У����ʵ�״̬����Ӧ�ȵ���ֵ�뵥λ����Ӧ�ȵ���ֵ�뻯ѧ����ʽǰ���ϵ�������ȣ���Ŀ�ѶȲ���

��ϰ��ϵ�д�
 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�
�����Ŀ
������Һһ�������Ե��ǣ�������
| A��pH=7����Һ |
| B��c��H+��=c��OH-������Һ |
| C���ǵ��������ˮ�õ�����Һ |
| D����ǿ�ᡢǿ������ʵ�����Ӧ�õ�����Һ |
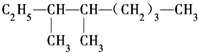 ��ϵͳ������������
��ϵͳ������������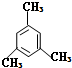 ��������
��������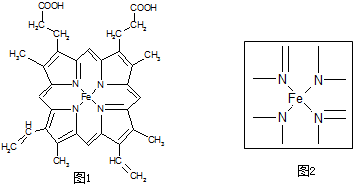
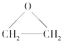 �����÷�Ӧ��ԭ��������Ϊ100%����Ӧ�Ļ�ѧ����ʽΪ
�����÷�Ӧ��ԭ��������Ϊ100%����Ӧ�Ļ�ѧ����ʽΪ