��Ŀ����
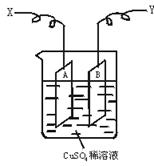
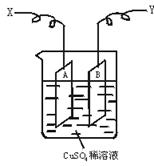
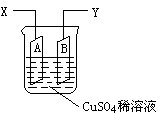
��ͼ�ǿ����ڲ��������ӵ�������װ��ʾ��ͼ������A��B�����鴿ͭƬ������CuSO4ϡ��Һ�У�ͭƬ���������������������˷ֱ�ΪX��Y��
��1������I=0.21 A�ĵ������60���Ӻ��ͭƬA������������0.25 g����ͼ4��4װ���е�X��Ӧ��ֱ�����__________�����������ǵ��ص�__________����
��2������ͭƬB������__________��ѡ����ӡ������١����䡱��
��3����ʽ����ʵ���õİ����ӵ�����NA������֪���ӵ����e��=1.60��10��19 C��
��������1���ɵ��ͭ�����֪ʶ����֪ͭƬA���������ӣ�˵��A����������֮������X��Ӧ��ֱ����ĸ���������
��2����B��������������Ӧ��Cu��2e��====Cu2+���ʵ���ͭƬB���������٣����ٵ��������ܡ���A�ϡ�
��3��ÿ����1 mol Cu����Ҫת��2 mol e����������0.25 g Cu����ת��2��![]() =0.0078 mol e����
=0.0078 mol e����
������ѧ֪ʶ֪����ϵ��ͨ���ĵ��ӵ����ʵ���Ϊn��e����=![]()
![]() =0.0078 mol��
=0.0078 mol��
NA=0.21 A��60��![]() =6.0��1023 mol��1
=6.0��1023 mol��1
�𰸣���1���� ��
��2������
��3��NA=![]() =6.0��1023 mol��1
=6.0��1023 mol��1

��ϰ��ϵ�д�
�����Ŀ