��Ŀ����
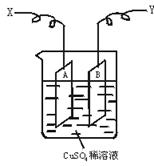
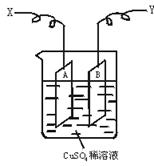
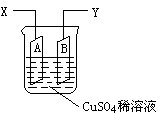
��ͼ�ǿ����ڲ��������ӵ�������װ��ʾ��ͼ������A��B�����鴿ͭƬ������CuSO4ϡ��Һ�У�ͭƬ���������������������˷ֱ�ΪX��Y��
(1)����I=
(2)����ͭƬB������____________��(����ӡ������١����䡱)
(3)��ʽ����ʵ���õİ����ӵ�����NA(��֪������e=1.60��10
��������ΪͭƬA���������ӣ�����AΪ���ص�������������ӦCu2++2e-![]() Cu��X��Ӧ��ֱ����Դ�ĸ��������ӡ�ͭƬB��Ϊ���ص�������Cu-2e-
Cu��X��Ӧ��ֱ����Դ�ĸ��������ӡ�ͭƬB��Ϊ���ص�������Cu-2e-![]() Cu2+�����������١�
Cu2+�����������١�
�𰸣�(1)�� �� (2)����
(3)![]() =6.0��1023 mol-1
=6.0��1023 mol-1

��ϰ��ϵ�д�
 �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д�
�����Ŀ