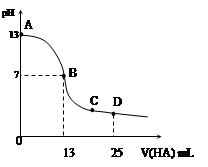��Ŀ����
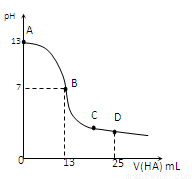
�����£���25 mL 0.1 mol/L MOH��Һ����μ���0.2 mol/L HA��Һ��������ͼ��ʾ������仯���Բ��ƣ����ش��������⣺
��1��д��MOH�ĵ��뷽��ʽ
��2��MOH��HAǡ����ȫ��Ӧʱ����Һ��_____�ԣ���ᡱ��������С����������ǣ������ӷ���ʽ��ʾ��__ _____��
��ʱ�������Һ����ˮ�������c(H+)__ _ 0.2 mol/L HA��Һ����ˮ�������c(H+)���������������=������
��3���ֱ�д��B��C���㣬�����Һ�и�����Ũ�ȵĴ�С��ϵ
B��_____________ _��C��___ __________��
��4��D��ʱ����Һ��c(A��)+c(HA)________2 c(M+)���������������=����������ʱ��û����Һ��pH = 3���� c(HA) + c(H+) = __________mol/L��
���𰸡�
��1��MOH==M++OH-��2���A��
+ H2O HA +
OH������
HA +
OH������
��3��c(M+) = c(A��)�� c(H+) = c(OH��) ��c(A��)��c(M+)�� c(H+)�� c(OH��) (��3��)
��4��= ��0.05���ɵ���غ�������غ���ã�
����������

��ϰ��ϵ�д�
�����Ŀ