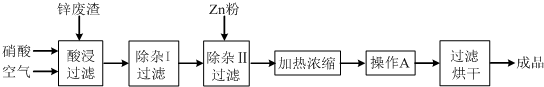
��Ŀ����
��18.4mol/L��ŨH2SO4����100mL 0.50mol/L��ϡH2SO4���밴Ҫ�����
��1������Ͳ��ȡ����ŨH2SO4�����Ϊ mL��
��2�����ʵ������10mL��20mL��50mL��Ͳ��Ӧѡ�� mL��Ͳ��ʵ���л���Ҫ�õ��������� �� �� ���ձ�����������
��3����ʵ���г������������������ҺŨ����ʲôӰ�죿����ƫ�ߡ�ƫ�͡���Ӱ�죩
��Ũ�����ܽ��δ�������¼����ж���
�ڶ���ʱ���ӿ̶���
������ƿϴ����δ���� ��
��ҡ�Ⱥ���Һ����ڿ̶��ߺ��ˮ����Һ�İ�Һ����̶�����ƽ ��
������Ͳ��ȡŨH2SO4ʱ���ӿ̶��ߣ� ��
��4��������ʱҺ����ڿ̶���Ӧ��ȡ�Ĵ�ʩ�� ��
��1������Ͳ��ȡ����ŨH2SO4�����Ϊ
��2�����ʵ������10mL��20mL��50mL��Ͳ��Ӧѡ��
��3����ʵ���г������������������ҺŨ����ʲôӰ�죿����ƫ�ߡ�ƫ�͡���Ӱ�죩
��Ũ�����ܽ��δ�������¼����ж���
�ڶ���ʱ���ӿ̶���
������ƿϴ����δ����
��ҡ�Ⱥ���Һ����ڿ̶��ߺ��ˮ����Һ�İ�Һ����̶�����ƽ
������Ͳ��ȡŨH2SO4ʱ���ӿ̶��ߣ�
��4��������ʱҺ����ڿ̶���Ӧ��ȡ�Ĵ�ʩ��
���㣺����һ�����ʵ���Ũ�ȵ���Һ
ר�⣺
��������1��������Һϡ�Ͷ���CŨVŨ=CϡVϡ�����㣻
��2�����ݡ����������ԭ������Ҫ��ȡ��Ũ����������ѡ����ʵ���Ͳ���������Ʋ����Ǽ��㡢��ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������Ҫ��������
��3������c=
��������ʵ����ʵ���n����Һ�����V�ı仯��������������
��4�����ƹ����еIJ���ʧ���ܲ��ȾͲ��ȣ����ܲ��Ⱦ����������ƣ�
��2�����ݡ����������ԭ������Ҫ��ȡ��Ũ����������ѡ����ʵ���Ͳ���������Ʋ����Ǽ��㡢��ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������Ҫ��������
��3������c=
| m |
| M |
��4�����ƹ����еIJ���ʧ���ܲ��ȾͲ��ȣ����ܲ��Ⱦ����������ƣ�
���
�⣺��1������ҪŨ��������ΪVmL��������Һϡ�Ͷ���CŨVŨ=CϡVϡ��֪��18.4mol/L��VmL=100mL��0.50mol/L
���V=2.7mL���ʴ�Ϊ��2.7��
��2�����ݡ����������ԭ������Ҫ��ȡ��Ũ��������Ϊ2.7mL����Ӧѡ��10mL����Ͳ�����������м��㡢��ȡ��ϡ�͡�ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ������Ͳ��ȡŨ���ᣬ���ձ���ϡ�ͣ�������Ͳ��ȡˮ�����ձ��������ò��������裮��ȴ��ת�Ƶ�100mL����ƿ�У����ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�����������������Ͳ���ձ�����������100mL����ƿ����ͷ�ιܣ��ʴ�Ϊ��10��100mL����ƿ����ͷ�ιܣ�
��3����Ũ�����ܽ��δ�������¼����ж��ݣ�����ȴ����Һ���ƫС����Ũ��ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
�ڶ���ʱ���ӿ̶��ߣ�����Һ���ƫС��Ũ��ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
��������ƿδ���T����������Һ������ҺŨ����Ӱ�죬��ΪֻҪ����ʱ��ȷ������ˮ��ԭ�����еĻ��Ǻ�������ģ���Ũ����Ӱ�죬�ʴ�Ϊ����Ӱ�죻
��ҡ�Ⱥ���Һ����ڿ̶����������ģ����ˮ����Һ�İ�Һ����̶�����ƽ������ҺŨ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
������Ͳ��ȡŨH2SO4ʱ���ӿ̶��ߣ��ᵼ����ȡ����Һ���ƫ��������ϡ�����Ũ��ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
��4��������ʱҺ����ڿ̶��ߣ������ȣ������������ƣ��ʴ�Ϊ���������ƣ�
���V=2.7mL���ʴ�Ϊ��2.7��
��2�����ݡ����������ԭ������Ҫ��ȡ��Ũ��������Ϊ2.7mL����Ӧѡ��10mL����Ͳ�����������м��㡢��ȡ��ϡ�͡�ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ������Ͳ��ȡŨ���ᣬ���ձ���ϡ�ͣ�������Ͳ��ȡˮ�����ձ��������ò��������裮��ȴ��ת�Ƶ�100mL����ƿ�У����ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�����������������Ͳ���ձ�����������100mL����ƿ����ͷ�ιܣ��ʴ�Ϊ��10��100mL����ƿ����ͷ�ιܣ�
��3����Ũ�����ܽ��δ�������¼����ж��ݣ�����ȴ����Һ���ƫС����Ũ��ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
�ڶ���ʱ���ӿ̶��ߣ�����Һ���ƫС��Ũ��ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
��������ƿδ���T����������Һ������ҺŨ����Ӱ�죬��ΪֻҪ����ʱ��ȷ������ˮ��ԭ�����еĻ��Ǻ�������ģ���Ũ����Ӱ�죬�ʴ�Ϊ����Ӱ�죻
��ҡ�Ⱥ���Һ����ڿ̶����������ģ����ˮ����Һ�İ�Һ����̶�����ƽ������ҺŨ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
������Ͳ��ȡŨH2SO4ʱ���ӿ̶��ߣ��ᵼ����ȡ����Һ���ƫ��������ϡ�����Ũ��ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
��4��������ʱҺ����ڿ̶��ߣ������ȣ������������ƣ��ʴ�Ϊ���������ƣ�
���������⿼����һ�����ʵ���Ũ����Һ�����ƹ����еļ���������������ڻ�������Ŀ���ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
���л�ѧʵ��������¹ʴ�����������ȷ���ǣ�������
| A���������ὦ�����У�Ӧ������ˮ��ϴ����ϴ��գ�۾� |
| B��������Ũ����Һմ��Ƥ���ϣ�Ҫ�����ô���ˮ��ϴ��Ȼ��Ϳ������ |
| C���ƾ����Ż�ʱ����ʪ������ |
| D������������Һʱ����������Ͳ�м���һ�������ˮ�����ڽ���������������Ũ���� |
���¶�ѹǿ���������£���֪N2O4?2NO2��2mol N2O4���ܱ������ڷֽ��NO2������ƽ��ʱ������0.6mol NO2����ƽ��ʱ����������ʵ����ǣ�������
| A��0.6mol |
| B��1.8mol |
| C��2.2mol |
| D��2.3mol |