题目内容
15.据报道,北京奥运会“祥云”火炬燃料为丙烷,悉尼奥运会火炬燃料为丁烷和丙烷混合气.标准状况下,1.0mol丙烷和丁烷的混合气和足量氧气混合完全燃烧后,恢复至原状态,混合气体的体积减小了70.0L,混合气体中丙烷和丁烷的体积比为3:1.分析 标准状况下,1.0mol丙烷和丁烷的混合气和足量氧气混合完全燃烧后,恢复至原状态,混合气体的体积减小了70.0L,物质的量为3.125mol;
设丙烷物质的量为X,丁烷物质的量为1-X;
C3H8(g)+5O2(g)→3CO2(g)+4H2O(l)△n
1 3
X 3X
2C4H10(g)+13O2(g)→8CO2(g)+10H2O(l)△n
2 7
1-X 3.5(1-X)
3X+3.5(1-X)=3.125
X=0.75mol,由此分析解答.
解答 标准状况下,1.0mol丙烷和丁烷的混合气和足量氧气混合完全燃烧后,恢复至原状态,混合气体的体积减小了70.0L,物质的量为3.125mol;
设丙烷物质的量为X,丁烷物质的量为1-X;
C3H8(g)+5O2(g)→3CO2(g)+4H2O(l)△n
1 3
X 3X
2C4H10(g)+13O2(g)→8CO2(g)+10H2O(l)△n
2 7
1-X 3.5(1-X)
3X+3.5(1-X)=3.125
X=0.75mol
所以混合气体中丙烷和丁烷的体积比=0.75:(1-0,75)=3:1,
故答案为:3:1.
点评 本题考查了阿伏伽德罗定律的计算应用,混合物的计算应用和判断,燃烧前后气体体积的变化计算,题目难度中等.

 数学奥赛暑假天天练南京大学出版社系列答案
数学奥赛暑假天天练南京大学出版社系列答案 南大教辅抢先起跑暑假衔接教程南京大学出版社系列答案
南大教辅抢先起跑暑假衔接教程南京大学出版社系列答案①煤的燃烧 ②工业废气的任意排放 ③燃放鞭炮 ④汽车尾气的排放.
| A. | ①② | B. | ①②③ | C. | ②④ | D. | ①②③④ |
| A. | SO2 | B. | CO2 | C. | NO2 | D. | PM2.5 |
| A. | 化学反应不一定都有能量变化 | |
| B. | 增大反应物浓度,活化分子百分数增大,化学反应速率一定增大 | |
| C. | 升高温度,活化分子百分数增大,化学反应速率一定增大 | |
| D. | 一般使用催化剂可以降低反应的活化能,增大活化分子百分数,从而提高反应物的转化率 |

| A. | 元素①位于第二周期第IVA族 | |
| B. | 元素的最高正价是③=⑤ | |
| C. | 最高价氧化物对应水化物的酸性:③>⑤>④ | |
| D. | 气态氢化物的稳定性:④<②<③ |
| A. | 溶剂蒸发快,得到的晶体颗粒也较大 | |
| B. | 减压过滤可过滤胶状沉淀物,且较为干燥 | |
| C. | 由0.1 mol•L-1一元碱BOH溶液的pH=10,可推知BOH溶液有BOH═B++OH- | |
| D. | 由0.1 mol•L-1一元酸HA溶液的pH=3,可推知NaA溶液有A-+H2O?HA+OH- |
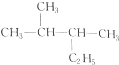 与
与
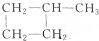 与
与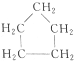
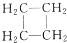 与
与