��Ŀ����
5���߷��Ӳ���PET������֬��PMMA�ĺϳ�·�����£�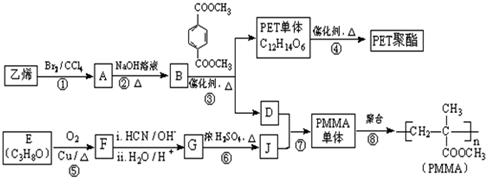
��֪��
��RCOOR��+R��18OH$��_{��}^{����}$RCO18OR��+R��OH��R��R�䡢R�����������
��
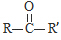 $\underset{\stackrel{i��HCN/O{H}^{-}}{��}}{ii��{H}_{2}O/{H}^{+}}$
$\underset{\stackrel{i��HCN/O{H}^{-}}{��}}{ii��{H}_{2}O/{H}^{+}}$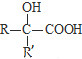 ��R��R�����������
��R��R�������������1���ٵķ�Ӧ�����Ǽӳɷ�Ӧ��
��2���ڵĻ�ѧ����ʽΪ
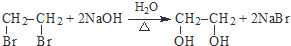 ��
����3��PMMA����Ĺ�����������̼̼˫����������
��4��F�ĺ˴Ź���������ʾֻ��һ��壬�ݵĻ�ѧ����ʽΪ
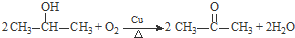 ��
����5��G�Ľṹ��ʽΪ
 ��
����6������˵����ȷ����ac������ĸ��ţ���
a����Ϊ������Ӧ
b��B��D��Ϊͬϵ��
c��D�ķе��̼ͬԭ������������
d��1mol
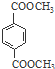 ������NaOH��Һ��Ӧʱ���������4mol NaOH
������NaOH��Һ��Ӧʱ���������4mol NaOH��7��д����PET�����Ʊ�PET��������ѧʽΪC10nH8nO4n��C10n+2H8n+6O4n+2��������B�Ļ�ѧ����ʽ
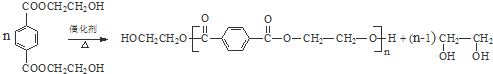 ��
��
���� ��ϩ���巢���ӳɷ�Ӧ����AΪCH2BrCH2Br��A��NaOHˮ��Һ�����������·���ˮ�ⷴӦ����BΪHOCH2CH2OH������Ϣ��PET����ķ���ʽ��֪��PET����Ϊ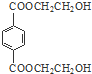 ����DΪCH3OH��C������Ϣ���н�����Ӧ���е����۷�Ӧ����PET��֬Ϊ
����DΪCH3OH��C������Ϣ���н�����Ӧ���е����۷�Ӧ����PET��֬Ϊ ����PMMA�Ľṹ����֪PMMA����ΪCH2=C��CH3��COOCH3����D��J��Ӧ�õ�PMMA���壬��JΪCH2=C��CH3��COOH��F������Ϣ���еķ�Ӧ�õ�G��G��Ũ���������·�����ȥ��Ӧ����J����EΪ
����PMMA�Ľṹ����֪PMMA����ΪCH2=C��CH3��COOCH3����D��J��Ӧ�õ�PMMA���壬��JΪCH2=C��CH3��COOH��F������Ϣ���еķ�Ӧ�õ�G��G��Ũ���������·�����ȥ��Ӧ����J����EΪ ��FΪ
��FΪ ��GΪ
��GΪ ��
��
��� �⣺��ϩ���巢���ӳɷ�Ӧ����AΪCH2BrCH2Br��A��NaOHˮ��Һ�����������·���ˮ�ⷴӦ����BΪHOCH2CH2OH������Ϣ��PET����ķ���ʽ��֪��PET����Ϊ ����DΪCH3OH��C������Ϣ���н�����Ӧ���е����۷�Ӧ����PET��֬Ϊ
����DΪCH3OH��C������Ϣ���н�����Ӧ���е����۷�Ӧ����PET��֬Ϊ ����PMMA�Ľṹ����֪PMMA����ΪCH2=C��CH3��COOCH3����D��J��Ӧ�õ�PMMA���壬��JΪCH2=C��CH3��COOH��F������Ϣ���еķ�Ӧ�õ�G��G��Ũ���������·�����ȥ��Ӧ����J����EΪ
����PMMA�Ľṹ����֪PMMA����ΪCH2=C��CH3��COOCH3����D��J��Ӧ�õ�PMMA���壬��JΪCH2=C��CH3��COOH��F������Ϣ���еķ�Ӧ�õ�G��G��Ũ���������·�����ȥ��Ӧ����J����EΪ ��FΪ
��FΪ ��GΪ
��GΪ ��
��
��1���ٵķ�Ӧ�����Ǽӳɷ�Ӧ���ʴ�Ϊ���ӳɷ�Ӧ��
��2���ڵĻ�ѧ����ʽΪ��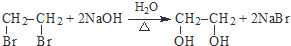 ��
��
�ʴ�Ϊ��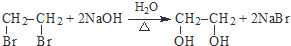 ��
��
��3��PMMA����ΪCH2=C��CH3��COOCH3��PMMA����Ĺ����������ǣ�̼̼˫��������2��
�ʴ�Ϊ��̼̼˫����������
��4���ݵĻ�ѧ����ʽΪ��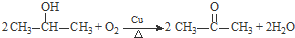 ��
��
�ʴ�Ϊ��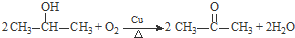 ��
��
��5��G�Ľṹ��ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��6��a������CH2=C��CH3��COOH��״�����������Ӧ����CH2=C��CH3��COOCH3����a��ȷ��
b��BΪHOCH2CH2OH��DΪCH3OH�����ߺ����ǻ���Ŀ��ͬ������ͬϵ���b����
c��DΪCH3OH������֮���γ�������е���ڼ���ģ���c��ȷ��
d��1mol ������NaOH��Һ��Ӧʱ���������2mol NaOH����d����
������NaOH��Һ��Ӧʱ���������2mol NaOH����d����
�ʴ�Ϊ��ac��
��7����PET�����Ʊ�PET��������ѧʽΪC10nH8nO4n��C10n+2H8n+6O4n+2��������B�Ļ�ѧ����ʽ�� ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���л�����ƶ���ϳɣ�������ø������Ϣ���л���Ľṹ�����ƶϣ���Ҫѧ���������չ����ŵ�������ת�����ϺõĿ���ѧ����ѧ������֪ʶǨ�����ã��Ѷ��еȣ�

| A�� | Al2S3 | B�� | SO2 | C�� | H2SiO3 | D�� | NO2 |
| ���� | 1 | 2 | 3 |
| ����/mL | 19.98 | 20.02 | 19.00 |
��2�������������漰�ķ�Ӧ����2Fe3++2I-�T2Fe2++I2��6Fe2++Cr2O72-+14H+�T6Fe3++2Cr3++7H2O��
��3�����ݵζ��й����ݣ��÷�Һ��I-������15.24g•L-1��
��4���ڵζ������У����в���������������ȷ������ɲⶨ���ƫ�͵���A��
A���յ����ʱ���Ӷ������ζ�ǰƽ�Ӷ���
B����ƿˮϴ�º�δ����
C���ζ���δ�ñ�K2Cr2O7��Һ��ϴ
D��ʢ��K2Cr2O7��Һ�ĵζ��ܣ��ζ�ǰ�����ݣ��ζ��������ݣ�
��Fe3+��Ag+�����������ǿ��һֱ��ʵ��̽�����ȵ㣮ijѧϰС��ͬѧ�������ʵ�飺
| ʵ���� | ʵ����� | ���� |
| 1 | ��10mL 3mol/L KNO3������Һ��pH=1���в���һ���ྻ��Ag˿�����μ�NaCl��Һ | �ް�ɫ�������� |
| 2 | ��10mL 1mol/L AgNO3��Һ�еμ�2mL 0.1mol/L FeSO4��Һ�����ٵμ�����KMnO4��Һ | �Ϻ�ɫ����ȥ |
| 3 | ��10mL 1mol/L Fe��NO3��3������Һ��pH=1���в���һ���ྻ��Ag˿�����μ�NaCl��Һ | �а�ɫ�������� |
��5�����ʵ��ٵ�Ŀ�����ų�NO3-�ĸ��ţ�
��6��ʵ��ۿɵó�������Fe3+������Ag��
��7��д��ʵ����з�Ӧ�����ӷ���ʽFe2++Ag+?Fe3++Ag��
��8����������ʵ�飬Fe3+��Ag+�����������ǿ��������Ũ���йأ�
 ��ͼ��ʢ��CCl4��U�ι��������˷ֱ���뱥��ʳ��ˮ��ϡ���ᣬʹ����Һ����ƽ�������ϲ�����˿�����ӣ��ܷ�ã�����һ��ʱ�䣮����˵��������ǣ�������
��ͼ��ʢ��CCl4��U�ι��������˷ֱ���뱥��ʳ��ˮ��ϡ���ᣬʹ����Һ����ƽ�������ϲ�����˿�����ӣ��ܷ�ã�����һ��ʱ�䣮����˵��������ǣ�������| A�� | ����������˿��ʴ����һ���� | B�� | �������߸�����Ӧ��ΪFe-2e��Fe2+ | ||
| C�� | ���Һ������ұ�Һ�� | D�� | ����������Һ��pH������ |
| A�� | ȡ25gCuSO4•5H2O����1Lˮ�� | |
| B�� | ��CuSO4•5H2O����ȥ���ᾧˮ��ȡ16g����ˮ�Ƴ�1L��Һ | |
| C�� | ��25gCuSO4•5H2O����ˮ�Ƴ�1L��Һ | |
| D�� | ��12.5gCuSO4•5H2O����500mLˮ�� |



 ��E��F�ķ�Ӧ����Ϊȡ����Ӧ��
��E��F�ķ�Ӧ����Ϊȡ����Ӧ�� +
+ $\stackrel{һ������}{��}$
$\stackrel{һ������}{��}$ +HCl��
+HCl�� ��д�ṹ��ʽ����
��д�ṹ��ʽ���� �ͣ�CH2��3CClΪ��ʼԭ���Ʊ�
�ͣ�CH2��3CClΪ��ʼԭ���Ʊ� �ĺϳ�·��
�ĺϳ�·�� ��
��
 ��
�� ��
�� ��
��